
Chimeric antigen receptor – CAR T Cell therapy represents a groundbreaking approach in cancer treatment, that uses the power of the body’s immune system to combat the disease. This innovative therapy involves genetically modifying a patient’s T cells, a type of white blood cell, to recognize and attack cancer cells more effectively. By equipping these cells with a synthetic receptor called a chimeric antigen receptor (CAR), CAR T-cell therapy has shown remarkable success in treating certain types of cancers, particularly blood cancers.
This image is taken from mskcc.org
CAR T-cell therapy is a form of immunotherapy that utilizes the patient’s own T cells, which are a crucial component of the immune system responsible for fighting infections and diseases. In this therapy, T cells are collected from the patient’s blood and genetically engineered in a laboratory to express a CAR on their surface. This CAR is designed to recognize and bind to specific proteins (antigens) found on the surface of cancer cells.
Once the CAR T cells are infused back into the patient’s body, they can identify and attach to the targeted cancer cells, triggering a potent immune response. The CAR T cells then proliferate and initiate a series of immune responses aimed at destroying the cancer cells.
CAR T cell therapy is an exciting new treatment that uses your own immune system to fight cancer. This therapy involves changing your T cells, a type of white blood cell, so they can better find and destroy cancer cells. Here’s a simple explanation of how it works:
The key to CAR T cell therapy is changing your T cells to have special receptors called chimeric antigen receptors (CARs) on their surface. Think of these CARs as tiny keys that can lock onto specific proteins, or antigens, on the surface of cancer cells. This helps the CAR T cells recognize and attach to cancer cells, which they might not be able to do on their own.
Once the CAR T cells attach to the cancer cells, they get activated. This activation is like turning on a switch that makes the T cells ready to fight. The CAR T cells then use several methods to kill the cancer cells:
Perforin and Granzyme Pathway: The CAR T cells release a protein called perforin that makes tiny holes in the cancer cell’s surface. Through these holes, they send in enzymes called granzymes that cause the cancer cell to self-destruct.
Fas-Fas Ligand Interaction: The CAR T cells can also have a protein called Fas ligand (FasL) that attaches to a receptor on the cancer cells. This attachment sends a signal to the cancer cells to die.
Cytokine Release: The CAR T cells release signaling proteins called cytokines, which help boost the immune response against the cancer cells. These cytokines can also change the environment around the tumor to make it harder for cancer cells to survive and easier for immune cells to attack.
One of the amazing things about CAR T cell therapy is that these modified T cells can stay in your body and keep working for a long time. After the initial treatment, the CAR T cells can continue to multiply and patrol your body, looking for and destroying any remaining cancer cells. This ongoing activity helps keep the cancer from coming back.
Cancer cells often find ways to hide from the immune system, such as reducing the proteins that T cells recognize or creating a suppressive environment. CAR T cells are designed to overcome these tricks through several strategies:
MHC*-Independent Recognition: Unlike regular T cells that need a specific molecule (MHC) to recognize antigens, CAR T cells can directly bind to antigens on cancer cells without needing MHC. This allows them to target cancer cells that have reduced MHC molecules to escape detection.
Enhanced Trafficking and Infiltration: CAR T cells can be engineered to have receptors that help them move to and enter tumor sites more effectively. This makes them better at finding and killing cancer cells within solid tumors.
Combination Therapies: CAR T cells can be used with other treatments, such as checkpoint inhibitors, to enhance their effectiveness. Checkpoint inhibitors block the signals that cancer cells use to suppress the immune response, thereby boosting the activity of CAR T cells.
*MHC- Major histocompatibility complex, a group of protein antigens found in all nucleated cells of the body.
This informative and fascinating video is taken from the Peter MacCallum Cancer Center
The process of CAR T cell therapy, a groundbreaking approach in the treatment of certain cancers, involves three critical stages: collecting, making, and infusing. This innovative treatment harnesses the patient’s own immune system to fight cancer more effectively.
The first stage in the CAR T cell therapy process is the collection of T cells from the patient’s blood. This is achieved through a procedure known as leukapheresis, which involves drawing blood from the patient. The blood is then processed to isolate T cells, which can be engineered and injected back into the patient to target and destroy cancer cells.
Once the T cells are collected, they are transported to a laboratory where they undergo a transformation to become CAR T cells. In this making stage, the collected T cells are genetically modified to express chimeric antigen receptors (CARs) on their surface. These receptors are designed to recognize and bind to specific proteins found on the surface of cancer cells. The genetic modification is achieved through the use of viral vectors, which insert the gene for the CAR into the T cells. After this modification, the T cells are expanded in the laboratory, growing millions of these engineered cells ready to be infused back into the patient.
The final stage of the CAR T cell therapy process is the infusion of the engineered CAR T cells back into the patient’s bloodstream. Before this infusion, patients may undergo a conditioning treatment, which often involves chemotherapy, to prepare their body to receive the CAR T cells. This conditioning helps create a more favorable environment for the CAR T cells to proliferate and perform their function. Once infused, the CAR T cells begin to seek out and destroy the cancer cells by binding to the specific proteins they were engineered to target. This targeted approach allows for the destruction of cancer cells while sparing healthy cells, marking a significant advancement in cancer treatment.
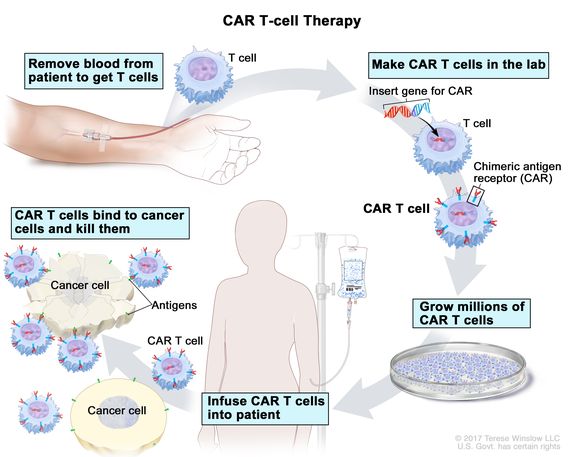
CAR T Cell therapy process; This image is taken from the cancer.gov
Several CAR T-cell therapies have received approval from regulatory agencies, such as the U.S. Food and Drug Administration (FDA), for the treatment of specific types of cancers.
Here’s a listing of the FDA-approved CAR T cell therapies, along with the types of cancer they’re used to treat. This information can help you understand the options available for certain blood cancers.
CAR T cell therapy in the use of different Cancer Types
Kymriah (tisagenlecleucel)
• Approved for children and young adults up to age 25 with B-cell precursor acute lymphoblastic leukemia (ALL) that is refractory or in second or later relapse.
• Also approved for adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL).
Yescarta (axicabtagene ciloleucel)
• Approved for adults with relapsed or refractory large B-cell lymphoma, including DLBCL, primary mediastinal large B-cell lymphoma, high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma.
• Also approved for adults with relapsed or refractory follicular lymphoma after two or more lines of systemic therapy.
Tecartus (brexucabtagene autoleucel)
• Approved for adults with relapsed or refractory mantle cell lymphoma.
• Also approved for adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
Breyanzi (lisocabtagene maraleucel)
• Approved for adults with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including DLBCL not otherwise specified, high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3B.
Abecma (idecabtagene vicleucel)
• Approved for adults with relapsed or refractory multiple myeloma after four or more prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody.
Carvykti (ciltacabtagene autoleucel)
• Approved for adults with relapsed or refractory multiple myeloma after four or more prior lines of therapy.
These therapies represent a significant advancement in cancer treatment, offering hope to patients with specific types of blood cancer. Each therapy is designed to target cancer cells in a precise way, helping the body’s immune system fight the disease more effectively.
If you or a loved one are considering CAR T cell therapy, it’s important to discuss with your healthcare provider which option might be the best fit based on the specific cancer type and individual health circumstances.
While CAR T-cell therapy has shown remarkable efficacy in treating certain cancers, it can also cause significant side effects. Some of the most common side effects include:
Cytokine release syndrome (CRS): This is a potentially life-threatening condition caused by the rapid release of cytokines (signaling proteins) by the activated CAR T cells. Symptoms of CRS can range from mild fever and flu-like symptoms to more severe complications such as low blood pressure, respiratory distress, and organ dysfunction. Medications like tocilizumab (Actemra) and corticosteroids are often used to manage CRS.
Neurological toxicities: CAR T-cell therapy can also cause neurological side effects, collectively known as immune effector cell-associated neurotoxicity syndrome (ICANS) or CAR T-cell-related encephalopathy syndrome (CRES). Symptoms may include confusion, delirium, seizures, and impaired consciousness. These neurological toxicities are typically managed with supportive care and, in severe cases, corticosteroids or other immunosuppressive agents.
Cytopenias: CAR T-cell therapy can lead to a decrease in the levels of various blood cells, including red blood cells (anemia), white blood cells (neutropenia), and platelets (thrombocytopenia). This can increase the risk of infections, bleeding, and fatigue.
Infections: Due to the suppression of the immune system during the conditioning regimen and the potential for prolonged cytopenias, patients receiving CAR T-cell therapy are at an increased risk of developing infections. Prophylactic antibiotics and antiviral medications may be prescribed to reduce this risk.
Tumor lysis syndrome (TLS): In some cases, the rapid destruction of cancer cells by the CAR T cells can lead to the release of large amounts of cellular debris, which can overwhelm the body’s ability to eliminate these byproducts. This can result in metabolic disturbances and organ damage, known as tumor lysis syndrome (TLS).
It is crucial for patients undergoing CAR T-cell therapy to be closely monitored and managed by experienced healthcare professionals in specialized treatment centers. Early recognition and prompt management of side effects are essential for ensuring the safety and success of the therapy.
In conclusion, CAR T cell therapy has revolutionized the treatment of certain cancers by harnessing the power of the immune system. Continued research and technological advancements hold the promise of further improving this therapy, making it more effective and accessible to a broader range of patients. As we look to the future, CAR T cell therapy and other immunotherapies are poised to play an increasingly important role in the fight against cancer and other diseases, offering new hope and improved outcomes for patients worldwide.
CAR T-Cell Therapy: Adverse Events and Management – PMC – NCBI
CAR T-cell Therapy and Its Side Effects | American Cancer Society- cancer.org
Next generations of CAR-T cells – new therapeutic opportunities in …
CAR T therapies in multiple myeloma: unleashing the future – Nature
Harnessing the potential of CAR-T cell therapy
Current status and future prospects of chimeric antigen receptor-T …
Recent advances and future perspectives of CAR-T cell therapy in …
CAR T Cell Therapy: A Game Changer in Cancer Treatment – PMC
CAR-T: What Is Next? – PMC – NCBI
Overview of approved CAR‐T therapies, ongoing clinical trials, and …
From bench to bedside: the history and progress of CAR T cell therapy
Exploring CAR-T Cell Therapy Side Effects: Mechanisms and … – MDPI
Side Effects | Stanford Health Care – stanfordhealthcare.org
Complete spectrum of adverse events associated with chimeric …
CAR-T cell therapy: current limitations and potential strategies – Nature
CAR T-cell therapy – Cancer Research UK
Long-term outcomes following CAR T cell therapy – Nature
Evolving CAR-T-Cell Therapy for Cancer Treatment – MDPI
CAR-T Cells: Future Perspectives – PMC – NCBI
The article is taken from OncoDaily
About IMMONC
Immune Oncology Research Institute (IMMONC) is dedicated to advancing research aimed at preventing, treating, and ultimately curing cancer while making these innovations accessible to those who need them. If you're interested in joining our team, please feel free to contact us at [email protected] or at +374-41 310-048.