Immunotherapy is emerging as a significant treatment option for colon cancer, particularly for patients with advanced or metastatic forms of the disease. This approach enhances the body’s immune system, enabling it to identify and attack cancer cells more effectively. Traditionally, colon cancer has been treated through surgery, chemotherapy, and radiation, but immunotherapy now offers an innovative alternative, especially for patients with specific genetic markers like microsatellite instability-high (MSI-H) or mismatch repair deficiency (dMMR).
This article will explore various types of immunotherapy used for colon cancer, including checkpoint inhibitors such as pembrolizumab and nivolumab. It will also discuss their success rates and potential side effects, including fatigue and immune-related adverse events. Clinical trials have shown promising survival benefits for patients with MSI-H colon cancer, providing hope for improved outcomes in this challenging disease.
Immunotherapy is an innovative approach to treating cancer by boosting the body’s immune system to recognize and attack cancer cells. In the context of colon cancer, immunotherapy, particularly immune checkpoint inhibitors, plays a key role for patients with advanced or metastatic disease, especially those with mismatch repair deficiency (dMMR) or microsatellite instability-high (MSI-H) tumors.
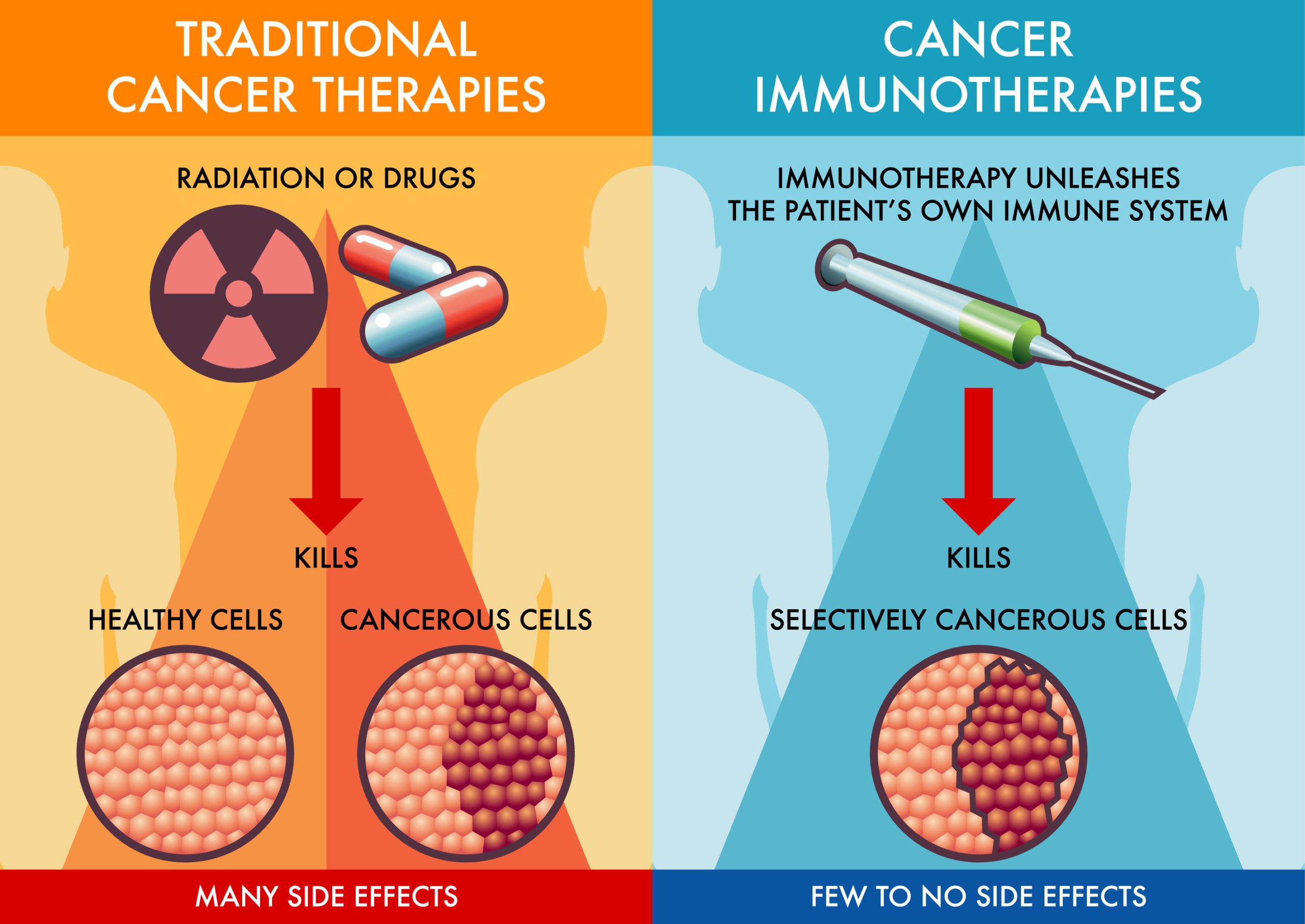
Mechanism of Action
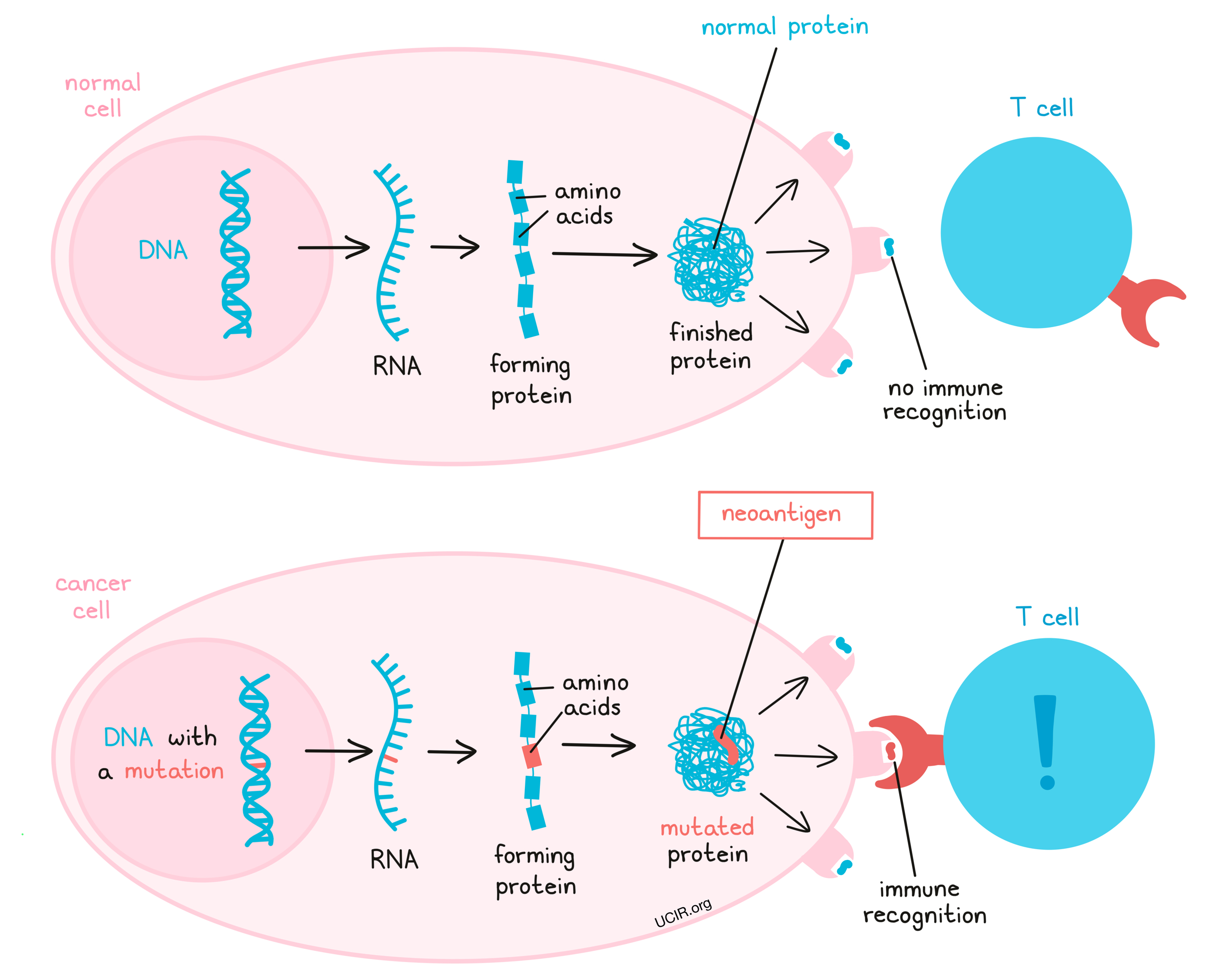
Immune checkpoint inhibitors target proteins like PD-1 or PD-L1 found on immune cells and cancer cells, respectively. These proteins typically regulate immune responses, preventing the immune system from attacking healthy cells. Cancer cells can exploit this mechanism to evade detection. By blocking PD-1 or PD-L1, drugs such as Pembrolizumab (Keytruda), Nivolumab (Opdivo), Durvalumab, Balstilimab, effectively “release the brakes” on T-cells, allowing the immune system to recognize and destroy cancer cells more efficiently.
Immunotherapy for colon cancer includes several approaches that enhance the immune system’s ability to fight cancer:
Checkpoint Inhibitors: These drugs block proteins like PD-1 or PD-L1, which cancer cells use to evade the immune system. In colon cancer, checkpoint inhibitors like pembrolizumab (Keytruda) are used for patients with microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) tumors, which respond better to immunotherapy.
Monoclonal Antibodies: These are lab-made antibodies designed to target specific antigens on cancer cells. For colon cancer, monoclonal antibodies such as bevacizumab (Avastin) target VEGF to prevent tumor blood vessel growth, improving outcomes when combined with chemotherapy.
Cancer Vaccines: Cancer vaccines are still under investigation for colon cancer. These aim to stimulate the immune system to attack cancer cells by presenting them with specific antigens found on colon cancer cells.
CAR T-Cell Therapy: This therapy involves engineering a patient’s T-cells to target specific antigens on cancer cells. While still experimental for colon cancer, CAR T-cell therapy is being explored in clinical trials to determine its effectiveness for this solid tumor.
Pembrolizumab (Keytruda) and Nivolumab (Opdivo) are immunotherapy drugs known as checkpoint inhibitors that work by targeting the PD-1 (programmed death-1) receptor on T-cells. Cancer cells often overexpress the protein PD-L1, which binds to PD-1 on T-cells and effectively turns off the immune response, allowing the tumor to grow unchecked. By blocking the PD-1/PD-L1 interaction, pembrolizumab and nivolumab help “release the brakes” on T-cells, enabling them to attack and destroy cancer cells more effectively.
Use in Colon Cancer
In colon cancer, particularly for patients with microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) tumors, pembrolizumab and nivolumab have shown promising results. These specific tumor types tend to have many genetic mutations, making them more recognizable to the immune system, thus enhancing the effectiveness of immunotherapy.
Success Rates
• Pembrolizumab: According to the KEYNOTE-177 trial, pembrolizumab as a first-line treatment for metastatic MSI-H/dMMR colorectal cancer resulted in a 40% response rate and improved progression-free survival compared to chemotherapy. At the 24-month mark, 48.3% of patients receiving pembrolizumab were still alive and progression-free, compared to 18.6% with standard chemotherapy.
• Nivolumab: Similarly, in the CheckMate-142 trial, nivolumab demonstrated a response rate of around 31% in previously treated patients with MSI-H/dMMR colorectal cancer. When combined with ipilimumab, the response rate increased to around 55%, highlighting the potential benefits of combination therapy in colon cancer treatment.
• Ipilimumab and Nivolumab: Nivolumab plus ipilimumab has shown impressive response rates in patients with microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) metastatic colorectal cancer. In the phase III CheckMate 8HW trial, the combination demonstrated a statistically significant and clinically meaningful improvement in progression-free survival compared to chemotherapy as first-line treatment, with a hazard ratio of 0.21 (p<0.0001)2. The objective response rate was 69% (95% CI, 53-82%) with a 13% complete response rate in the phase II CheckMate 142 study4. In the neoadjuvant setting, the NICHE-2 study reported pathological responses in 98% of patients with locally advanced dMMR colon cancer after only 4 weeks of treatment, including a major pathological response in 95% and a pathological complete response in 68% (According to Lenz et al study published in Journal of Clin Oncology in 2021)
• Botensilimab and Balstilimab: Balstilimab plus botensilimab has shown promising response rates in patients with microsatellite stable (MSS) colorectal cancer (CRC), which has historically been resistant to immunotherapy. In the phase 1 trial NCT03860272, the combination demonstrated an overall response rate (ORR) of 23% in heavily pretreated patients with MSS metastatic CRC. The 12- and 18-month overall survival rates were 71% and 62%, respectively, with a median overall survival of 21.2 months. In the neoadjuvant setting, the phase 2 NEST-1 trial reported even more impressive results, with a pathological response rate of 67.5% in patients with localized MSS CRC6. Notably, 63% of MSS patients (5/8) in NEST-1 achieved a pathological response (≥50% tumor regression) with just one dose of botensilimab and two doses of balstilimab, including one complete pathological response3. The combination also showed activity in mismatch repair deficient (dMMR)/microsatellite instability-high (MSI-H) CRC, with a 100% pathological response rate in this subgroup. These response rates are particularly significant given the historically poor outcomes of immunotherapy in MSS CRC, which represents about 90% of all CRC cases.(According to Kassi et al study published in Journal of clinical Oncology in 2024)
There are various monoclonal antibodies used to treat colorectal cancer including panitumumab, cetuximab, trastuzumab, ramucirumab and others.
• Cetuximab (Erbitux) and panitumumab (Vectibix) target the epidermal growth factor receptor (EGFR). Cetuximab has shown response rates of 23% to 34% in clinical trials for KRAS wild-type metastatic colorectal cancer. It’s used in about 40% of metastatic colorectal cancer treatments. Panitumumab improves overall survival and progression-free survival rates compared to chemotherapy alone in patients with wild-type RAS metastatic colorectal cancer.
• Ramucirumab (Cyramza) is another VEGF inhibitor approved for metastatic colorectal cancer. In the RAISE trial, it demonstrated a median overall survival of 13.3 months versus 11.7 months for placebo when combined with FOLFIRI chemotherapy. It’s extensively used in second-line treatment for metastatic colorectal cancer2.
• Monoclonal antibodies, such as trastuzumab (Herceptin), are targeted therapies that have shown promise in treating HER2-positive colon cancer, a subtype of colorectal cancer where the HER2 protein is overexpressed. HER2 is a growth factor receptor that, when overexpressed, leads to aggressive tumor growth. While trastuzumab is more commonly associated with breast cancer, it has been explored for HER2-positive colon cancer with encouraging results. By binding to this receptor, trastuzumab blocks the signaling pathways that promote cell proliferation and survival. In addition to blocking HER2, trastuzumab also engages the immune system by activating antibody-dependent cellular cytotoxicity (ADCC), where immune cells target and destroy the HER2-expressing cancer cells.
Effectiveness in HER2-Positive Colon Cancer
HER2 overexpression occurs in about 2-3% of colorectal cancers, particularly in tumors that do not have mutations in KRAS or NRAS genes. This makes a small subset of patients eligible for HER2-targeted therapies like trastuzumab.
Several clinical trials have evaluated trastuzumab in combination with other drugs for HER2-positive colorectal cancer:
• HERACLES Trial: One of the most notable studies, the HERACLES trial, investigated trastuzumab in combination with lapatinib (a HER2 kinase inhibitor) in patients with HER2-positive metastatic colorectal cancer. The trial showed promising results, with 30% of patients experiencing a partial response, and another 44% showing stable disease.
• MOUNTAINEER Trial: Another study looked at trastuzumab combined with tucatinib, another HER2 inhibitor, in HER2-positive colorectal cancer. Preliminary data showed an objective response rate (ORR) of around 50% in patients with metastatic disease, indicating a significant benefit for HER2-targeted therapies in colon cancer.
Cancer vaccines are an emerging area of immunotherapy focused on stimulating the immune system to recognize and attack cancer cells. In the context of colon cancer, the development of therapeutic vaccines is still in the early stages, but significant progress is being made.
Unlike preventive vaccines, such as those for HPV, cancer vaccines are therapeutic and designed to treat existing cancers by enhancing the body’s immune response against tumor-associated antigens. In colon cancer, vaccines target specific proteins expressed by cancer cells, like carcinoembryonic antigen (CEA) and MUC1. These antigens help the immune system differentiate between healthy and cancerous cells, facilitating a more focused immune attack.
Current research is exploring various types of vaccines for colon cancer, including peptide-based vaccines, dendritic cell vaccines, and whole-cell vaccines. For instance, a phase II trial investigated a CEA peptide vaccine in patients with advanced colon cancer, revealing that some patients experienced immune responses associated with prolonged survival. Dendritic cell vaccines involve extracting these immune cells from patients, exposing them to tumor antigens, and reintroducing them to stimulate a robust immune response, showing promise in generating T-cell activity against colon cancer cells. Whole-cell vaccines, like GVAX, use entire cancer cells modified to boost immune stimulation, and early-phase trials indicate effectiveness in slowing disease progression when combined with immune checkpoint inhibitors.
While still in development, cancer vaccines offer potential benefits, such as targeted treatment that minimizes damage to healthy tissues and the possibility of durable immune responses, leading to prolonged remission. According to a study by Feng et al. (2017), a phase I/II trial of a MUC1-based vaccine demonstrated an immune response in 43% of treated patients, with some achieving stable disease for over six months. Despite these promising early findings, further robust clinical trials are needed to confirm the efficacy and long-term benefits of cancer vaccines for a broader range of patients with colon cancer.
CAR T-cell therapy is a promising treatment for various cancers, including colon cancer, though it has demonstrated more success in blood cancers. This therapy involves extracting a patient’s T-cells, genetically modifying them to express chimeric antigen receptors (CARs) that can identify and attack cancer cells, and then reinfusing these modified cells back into the patient.
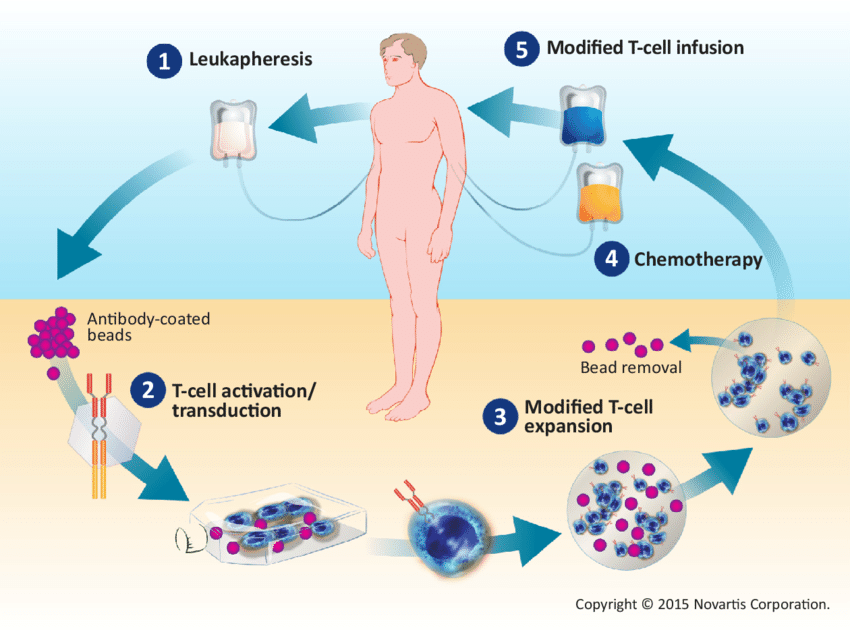
The mechanism targets specific proteins found on colon cancer cells, primarily carcinoembryonic antigen (CEA) and GUCY2C. By targeting these antigens, CAR T-cells can more effectively recognize and attack the cancer. The process includes collecting T-cells, modifying them to express CARs, and reinfusing them to multiply and combat the tumor.
However, CAR T-cell therapy faces challenges in solid tumors like colon cancer due to the tumor microenvironment, which often contains immunosuppressive cells and physical barriers that hinder T-cell infiltration. Early trials targeting CEA have shown modest results, prompting researchers to explore combination therapies with checkpoint inhibitors to enhance efficacy.
Current research focuses on improving CAR T-cells through combination approaches, developing “next-generation” T-cells that thrive in solid tumors, and targeting multiple antigens to reduce the risk of antigen escape.
Despite its potential, CAR T-cell therapy for colon cancer remains largely experimental, with challenges like cytokine release syndrome (CRS) and difficulties in penetrating solid tumors. Ongoing studies are essential to enhance its effectiveness and manage side effects for patients with advanced colorectal cancer.
The success rates of immunotherapy for colon cancer, particularly for microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) types, show promising outcomes. Key statistics include:
• Pembrolizumab (Keytruda): In the KEYNOTE-177 trial, pembrolizumab improved progression-free survival to 16.5 months in MSI-H/dMMR metastatic colon cancer compared to 8.2 months with chemotherapy. The overall response rate (ORR) was 44%, with some patients achieving long-term remission.
• Nivolumab (Opdivo): In combination with ipilimumab (Yervoy), nivolumab has shown an ORR of 55% in MSI-H/dMMR colon cancer, with durable responses in many cases.
For patients with non-MSI-H colon cancer, immunotherapy has not shown the same level of effectiveness, and research is ongoing to extend these therapies to a broader group of patients.
Immunotherapy has emerged as a highly effective treatment for microsatellite instability-high (MSI-H) colon cancer, which is characterized by defects in the DNA mismatch repair system. Patients with MSI-H colorectal cancer respond particularly well to immune checkpoint inhibitors, making this approach a key therapeutic option.
Checkpoint inhibitors like pembrolizumab (Keytruda) and nivolumab (Opdivo) have shown significant improvements in outcomes for treating MSI-H colorectal cancer. In the KEYNOTE-177 trial, pembrolizumab achieved a 2-year overall survival rate of 74%, compared to 65% for chemotherapy, with a median progression-free survival (PFS) of 16.5 months for pembrolizumab versus 8.2 months for chemotherapy. Nivolumab, whether used alone or in combination with ipilimumab (Yervoy), has also yielded promising results; patients receiving nivolumab alone had a 12-month overall survival rate of 73%, while those on the combination therapy had an 85% survival rate.
Immunotherapy often results in durable responses for patients with MSI-H colorectal cancer, allowing them to remain disease-free for extended periods even after treatment ends. Pembrolizumab, in particular, is associated with prolonged survival and long-term disease control. Furthermore, immunotherapy generally provides an improved quality of life compared to chemotherapy, as it tends to have fewer severe side effects. Common side effects, such as fatigue, diarrhea, and skin-related issues, are typically manageable, enhancing patients’ overall well-being during treatment.
Microsatellite Stable (MSS) Colon Cancer refers to colon cancer that does not exhibit high microsatellite instability (MSI), a condition where tumors are less likely to respond to immunotherapy treatments. However, improvements in chemotherapy, targeted therapies, and surgical interventions have led to better survival rates and quality of life for MSS colon cancer patients.
Success Rates for Microsatellite Stable (MSS) Colon Cancer
• Stage I-II MSS Colon Cancer: For early-stage MSS colon cancer, surgical resection is often curative. The 5-year survival rate is approximately 90% for stage I and 70-85% for stage II, depending on factors like tumor location and patient health.
• Stage III MSS Colon Cancer: Treatment typically involves surgery followed by adjuvant chemotherapy. The 5-year survival rate for stage III MSS colon cancer is around 50-75% when using chemotherapy regimens like FOLFOX (5-FU, leucovorin, and oxaliplatin).
• Stage IV MSS Colon Cancer: In the metastatic setting, outcomes are more challenging. The 5-year survival rate for stage IV MSS colon cancer ranges from 10-20%, though advancements in chemotherapy and targeted therapies, such as bevacizumab and cetuximab, have improved outcomes. Surgical interventions, like liver resections, also contribute to better survival rates.
Immunotherapy and Neoadjuvant Treatment
While traditional immunotherapy options have shown limited efficacy in MSS colon cancer, ongoing research is exploring its potential. Neoadjuvant immunotherapy may be utilized to shrink tumors before surgery, potentially enhancing the surgical outcome and improving survival rates. Current studies are focusing on combining immunotherapy with chemotherapy to improve overall effectiveness in MSS patients.
Quality of Life Improvements
Immunotherapy can offer several quality of life improvements for MSS colon cancer patients. It may result in fewer side effects than conventional chemotherapy, leading to a more tolerable treatment experience. Additionally, patients undergoing neoadjuvant immunotherapy might experience better disease control with reduced toxicity, allowing for improved physical function and emotional well-being. Enhanced management of treatment side effects, such as nausea and fatigue, can also contribute to an overall better quality of life.
According to multiple studies, including those published in CA: A Cancer Journal for Clinicians and Annals of Surgery, MSS colon cancer patients have seen improved survival outcomes through the combination of aggressive surgical interventions, chemotherapy, and targeted therapies, though responses to immunotherapies remain limited.
Colon cancer patients may experience side effects from treatment, such as fatigue, skin rash, and diarrhea. Serious side effects can include autoimmune reactions like colitis or hepatitis from immunotherapy. Effective monitoring and management of these effects are essential for maintaining treatment efficacy and quality of life.

Read Side effects of Immunotherapy Article by OncoDaily
In colon cancer treatment, common side effects, particularly from chemotherapy, include fatigue, nausea and skin issues. These side effects can vary in intensity depending on the type of treatment, individual patient responses, and overall health.
• Fatigue is one of the most frequently reported side effects of colon cancer treatment. It affects nearly all patients undergoing chemotherapy or radiation. Patients describe it as an overwhelming tiredness that doesn’t improve with rest. According to a study published in Cancer (2017), about 80-90% of colon cancer patients experience some degree of fatigue during treatment.
• Nausea and Vomiting: Chemotherapy for colon cancer, especially with drugs like oxaliplatin or irinotecan, commonly induces nausea and vomiting. Anti-nausea medications (antiemetics) are often prescribed to manage these symptoms. The prevalence of nausea can be high, affecting about 70-80% of patients receiving certain chemotherapy combinations, according to the Journal of Clinical Oncology.
• Skin Issues: Chemotherapy can lead to skin problems like dryness, rashes, and increased sensitivity to sunlight. This is particularly common with drugs like capecitabine and 5-FU (fluorouracil). Some patients also experience hand-foot syndrome, characterized by redness, swelling, and pain in the palms and soles. Around 20-30% of patients on capecitabine experience hand-foot syndrome, based on clinical trial data published in The Lancet Oncology.
Understanding and managing these side effects Through medication, lifestyle adjustments, and support systems can greatly improve a patient’s quality of life during colon cancer treatment.
Serious side effects of immunotherapy for colon cancer can include autoimmune reactions and infusion reactions. These adverse effects are a consequence of the immune system becoming overly activated, leading to unintended attacks on healthy tissue.
Autoimmune Reactions
Immune-related adverse events (irAEs) happen when the immune system starts attacking normal organs and tissues. This can affect various organs such as the liver, lungs, skin, and gastrointestinal tract.
• Colitis (inflammation of the colon) is a common irAE for patients receiving immunotherapy like checkpoint inhibitors. It presents as severe diarrhea, abdominal pain, and potential gastrointestinal bleeding.
• Hepatitis: Another serious side effect is autoimmune hepatitis, where the immune system targets the liver, causing inflammation. Patients may experience elevated liver enzymes and jaundice.
Infusion Reactions
Infusion reactions are another potential risk, typically occurring during or shortly after the infusion of the immunotherapy drugs. These reactions can range from mild (fever, chills) to severe (anaphylaxis).
• Mild Reactions: Most common symptoms include fever, chills, rash, or low blood pressure during the infusion. These symptoms are typically managed with antihistamines or corticosteroids.
• Severe Reactions: In rare cases, anaphylaxis, a life-threatening allergic reaction, can occur, causing difficulty breathing, swelling, and a drop in blood pressure.
Management of Side Effects
Managing these severe side effects often involves pausing treatment and administering immunosuppressive therapies such as corticosteroids to reduce inflammation. In some cases, immune-modulating drugs like infliximab may be used if first-line therapies are not effective.
Careful monitoring and early detection of these reactions are critical in minimizing the risks and allowing patients to safely continue with their treatment.
Immunotherapy for stage 4 colon cancer (metastatic) often involves a combination of chemotherapy, targeted therapies (like bevacizumab and cetuximab), and immunotherapy for eligible patients, with surgical resection considered for resectable metastases, enhancing outcomes alongside systemic therapy, and a study in the Journal of Clinical Oncology (2019) found that patients treated with surgery and targeted therapy had median survival rates of up to 30 months; despite the overall 5-year survival rate remaining around 14%, treatment advancements are improving life expectancy and quality of life.
Combination therapies, such as nivolumab (a PD-1 inhibitor) and ipilimumab (a CTLA-4 inhibitor), are particularly effective for patients with microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) tumors, as these tumors are more responsive to immune checkpoint inhibitors, and according to a Journal of Clinical Oncology (2020) study, this combination therapy demonstrated an objective response rate (ORR) of around 55% in MSI-H/dMMR patients, significantly improving progression-free survival (PFS) and overall survival (OS) compared to chemotherapy alone.
Single-agent immunotherapies, particularly nivolumab and pembrolizumab, show promise for patients with MSI-H/dMMR tumors, with nivolumab exhibiting a 31% response rate in these patients and a 12-month PFS rate of 50% (JCO, 2017), while pembrolizumab also significantly improved outcomes in the KEYNOTE-177 trial, demonstrating a 43.8% ORR and a median PFS of 16.5 months compared to 8.2 months with chemotherapy (NEJM, 2020), but these therapies are less effective in patients without these mutations, who typically receive standard chemotherapy or combination therapies.
The administration of immunotherapies for stage 4 colon cancer typically involves intravenous (IV) infusions, with specific schedules depending on the drug and the patient’s treatment plan. Below is an overview of the administration methods and typical schedules for the most commonly used single-agent immunotherapies:
Nivolumab
• Administration: Nivolumab is given as an IV infusion. The typical dose for colon cancer patients is 240 mg every two weeks or 480 mg every four weeks, depending on the patient’s overall health and physician preference.
• Duration: Each infusion lasts approximately 30–60 minutes.
• Frequency: Treatment is generally continued until disease progression or unacceptable toxicity, often over several months to years. According to clinical trials, some patients have received treatment for more than two years, particularly those who experience durable responses.
Pembrolizumab
• Administration: Pembrolizumab is also delivered via an IV infusion. The standard dosing schedule is 200 mg every three weeks or 400 mg every six weeks, depending on the specific regimen chosen.
• Duration: Each infusion lasts about 30 minutes.
• Frequency: Similar to nivolumab, pembrolizumab is continued until disease progression or adverse effects. Some patients remain on therapy for more than two years, particularly if they are showing sustained benefit.
Injection Schedules:
Immunotherapies like nivolumab and pembrolizumab are administered in a clinical setting under the supervision of healthcare professionals due to the risk of infusion reactions. Patients usually follow a schedule of every 2 to 6 weeks depending on the drug and response, and most receive regular follow-ups with oncologists to monitor efficacy and manage side effects.
• Initial Consultation: During the first consultation, the oncologist discusses the patient’s diagnosis, genetic markers (like MSI-H/dMMR), and treatment options. If immunotherapy is suitable, the plan, including drug choice and schedule, is outlined.
• Treatment Initiation: Immunotherapy, such as nivolumab or pembrolizumab, is administered via IV infusion in a clinical setting. Infusions typically last 30-60 minutes and occur every 2-6 weeks, depending on the regimen.
• Managing Side Effects: Common side effects include fatigue, rash, and diarrhea, which are monitored closely. Severe immune-related side effects, such as colitis or pneumonitis, require prompt management and may involve corticosteroids or therapy adjustments.
• Monitoring Progress: Patients undergo regular imaging scans and lab tests to assess the effectiveness of treatment. Progress is reviewed at follow-up visits, with adjustments made if necessary.
• Follow-Up Care: Long-term care involves continuous monitoring for both disease control and side effect management. Patients with a positive response may stay on therapy for months to years, depending on their overall health and tumor response.
The timeline for observing improvements after starting immunotherapy can vary by individual. Initially, patients may not notice significant changes in the first few weeks as the immune system is activated, with some experiencing mild side effects like fatigue or a rash. By 8-12 weeks, the first imaging scan typically assesses tumor response; some patients may show partial shrinkage or stable disease, while others might experience pseudo-progression. Significant improvements may become apparent by 3-6 months, with follow-up scans confirming tumor shrinkage or stabilization and potential symptom relief. For patients with durable responses, continued treatment beyond 6 months can lead to sustained remission, emphasizing the importance of regular monitoring to evaluate treatment effectiveness.
• Treatment Costs: Immunotherapies like nivolumab and pembrolizumab can be expensive, with annual costs reaching $100,000 to $150,000 or more, depending on the drug and duration of treatment.
• Insurance Coverage: Many insurance plans, including Medicare and private insurers, cover immunotherapy for patients with specific genetic markers like MSI-H/dMMR. However, coverage details can vary, and patients may face co-pays or deductibles.
• Financial Assistance: Pharmaceutical companies often provide patient assistance programs to reduce costs for eligible patients. Organizations like the Patient Advocate Foundation and CancerCare also offer financial support for treatment and related expenses.
Read more about Immunotherapy vs Chemotherapy Article by OncoDaily
Immunotherapy
Advantages: Effective for patients with MSI-H/dMMR tumors; can lead to long-term remission with fewer side effects than chemotherapy.
Limitations: Only works for certain genetic profiles; side effects can include immune-related adverse events. Not as effective for non-MSI-H/dMMR patients.
Surgery
Advantages: Can be curative in patients with resectable metastasis (e.g., liver or lung metastases). Improves survival when combined with other treatments.
Limitations: Only an option for a minority of patients with limited metastasis. Not suitable for widespread cancer.
Chemotherapy
Advantages: Effective for a broad range of patients; helps shrink tumors and slow progression. Regimens like FOLFOX or FOLFIRI are standard for stage 4 disease.
Limitations: Causes significant side effects (e.g., nausea, fatigue, hair loss); typically offers temporary control of disease with median survival of 12-30 months.
Targeted Therapy
Advantages: Works on specific mutations (e.g., KRAS, BRAF); fewer side effects than chemotherapy.
Limitations: Only useful for patients with specific genetic mutations; resistance may develop over time.
While immunotherapy offers promising long-term benefits, some patients face persistent health issues requiring continuous monitoring and care.
Durable Responses: Many patients, especially those with MSI-H/dMMR tumors, experience long-term tumor control or even remission. Immunotherapy can offer extended progression-free survival (PFS) and overall survival (OS) compared to traditional treatments.
Improved Quality of Life: Due to fewer severe side effects than chemotherapy, patients often maintain a better quality of life while on immunotherapy.
Immune-Related Side Effects: Long-term immunotherapy can lead to chronic autoimmune conditions, such as colitis, thyroid dysfunction, hepatitis, or pneumonitis, which may require lifelong management with medications like corticosteroids.
Fatigue and Weakness: Some patients report ongoing fatigue and muscle weakness even after completing treatment, affecting daily activities.
Financial Strain: Extended treatment duration, follow-up care, and managing side effects can result in continued financial burdens for patients and families.
Immunotherapy is an evolving treatment option for colon cancer, but it is primarily effective in specific types of the disease and for certain stages. While immunotherapy is most often used in stage 4 colon cancer, research is exploring its role in earlier stages and in combination with other treatments. The decision to use immunotherapy is based on several key factors. Here are the main criteria for determining a patient’s eligibility
Genetic Markers
The presence of microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) tumors is a critical factor. These tumors have a higher mutation load, making them more responsive to immune checkpoint inhibitors like nivolumab or pembrolizumab.
Stage of Cancer
Immunotherapy is most commonly used in advanced or metastatic (stage 4) colon cancer, where it has shown significant benefits. In earlier stages, it is only considered in cases of MSI-H/dMMR cancers or as part of clinical trials.
Previous Treatment Response
Patients whose cancers have not responded to standard treatments like chemotherapy or targeted therapies may be eligible for immunotherapy as a second-line or subsequent therapy.
Overall Health and Performance Status
The patient’s overall health and ECOG performance status are evaluated to ensure they can tolerate immunotherapy. Patients with autoimmune diseases may not be ideal candidates due to the risk of worsening their condition.
Clinical Trials
For patients who do not meet the typical eligibility criteria, participation in a clinical trial may be an option to access newer immunotherapy combinations or strategies.
Researchers are actively working to improve immunotherapy for colon cancer, particularly for patients who do not have MSI-H or dMMR tumors. These patients often do not respond well to current treatments, so the focus is on several key areas:
One approach is to combine immunotherapy with chemotherapy or targeted therapies. For instance, studies are testing the combination of pembrolizumab and FOLFOX chemotherapy for patients with stage 4 colon cancer, aiming to enhance treatment effectiveness.
In addition, new immune checkpoint inhibitors are being explored. These include drugs that target other immune pathways, such as LAG-3 and TIGIT inhibitors, which show promise in early trials.
Researchers are also investigating cancer vaccines and adoptive T-cell therapy. These treatments aim to boost the immune system’s ability to recognize and attack tumors. Early studies suggest they might help overcome the resistance some patients experience with existing immunotherapies.
Another critical area of research involves identifying new biomarkers. By studying genetic signatures and immune profiles, scientists hope to find ways to expand immunotherapy to a broader range of patients.
Maintaining a balanced diet is essential for patients undergoing immunotherapy. A variety of fruits, vegetables, whole grains, and lean proteins provides vital nutrients, while antioxidant-rich foods like berries and leafy greens can reduce inflammation and promote health.
Regular physical activity is beneficial; moderate exercise, such as walking or yoga, can improve immune function and alleviate fatigue. Staying well-hydrated is crucial, as adequate water intake helps maintain bodily functions and flush out toxins.
Managing stress through techniques like meditation and mindfulness enhances emotional well-being and supports immune health. While certain supplements, such as vitamin D, probiotics, and omega-3 fatty acids, may offer additional benefits, it’s important to consult a healthcare provider before starting any new supplements.
Finally, ensuring 7-9 hours of quality sleep each night supports recovery and immune function. These adjustments can help maintain a stronger immune system and overall health during immunotherapy, but always consult a healthcare provider before making changes.
What Is the Role of Immunotherapy in Treating Colon Cancer?
Immunotherapy enhances the body’s immune system to recognize and attack cancer cells. It is especially effective for patients with advanced or metastatic colon cancer, particularly those with microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) tumors.
How Do Checkpoint Inhibitors Work in Colon Cancer Treatment?
Checkpoint inhibitors like pembrolizumab (Keytruda) and nivolumab (Opdivo) block proteins such as PD-1 or PD-L1, which tumors use to evade immune detection. By blocking these proteins, the immune system is “unleashed” to attack the cancer cells more effectively.
Who Is Eligible for Immunotherapy in Colon Cancer?
Patients with advanced or metastatic colon cancer who have MSI-H or dMMR tumors are the best candidates for immunotherapy. For patients without these genetic markers, immunotherapy is still being explored in clinical trials.
What Are the Side Effects of Immunotherapy for Colon Cancer?
Common side effects include fatigue, skin rash, and diarrhea. More serious immune-related side effects, such as colitis or hepatitis, may occur and require prompt management with immunosuppressive drugs like corticosteroids.
Can Immunotherapy Be Used Alongside Chemotherapy for Colon Cancer?
Yes, ongoing research explores combining immunotherapy with chemotherapy. This combination may improve effectiveness in patients with both microsatellite stable (MSS) and MSI-H colon cancer.
How Effective Is Immunotherapy for Stage 4 Colon Cancer?
Immunotherapy has shown promising results for patients with MSI-H/dMMR tumors in stage 4 colon cancer. For example, pembrolizumab significantly improves progression-free survival compared to chemotherapy in these patients.
What Are the Long-Term Benefits of Immunotherapy for Colon Cancer Patients?
Immunotherapy can lead to durable responses, where patients remain disease-free for long periods, potentially experiencing prolonged survival. However, some may face chronic side effects requiring ongoing care.
Is CAR T-Cell Therapy an Option for Colon Cancer?
CAR T-cell therapy is still experimental for solid tumors like colon cancer. Early trials targeting specific antigens like CEA have shown modest results, but this therapy is mainly used for blood cancers.
How Do Monoclonal Antibodies Like Cetuximab Work in Colon Cancer Treatment?
Monoclonal antibodies like cetuximab target specific proteins on cancer cells. In colon cancer, cetuximab targets the epidermal growth factor receptor (EGFR), helping slow tumor growth and improve patient outcomes.
Can Patients with MSI-H Colon Cancer Achieve Long-Term Remission with Immunotherapy?
Yes, patients with MSI-H colon cancer have shown durable responses to immunotherapy. Clinical trials have demonstrated long-term remission in some cases, with ongoing monitoring required to ensure the treatment continues to work effectively.
The article is taken from OncoDaily.
About IMMONC
Immune Oncology Research Institute (IMMONC) is dedicated to advancing research aimed at preventing, treating, and ultimately curing cancer while making these innovations accessible to those who need them. If you're interested in joining our team, please feel free to contact us at [email protected] or at +374-41 310-048.