
Immunotherapy for breast cancer is gaining recognition as an innovative treatment for breast cancer, particularly for hard-to-treat subtypes like triple-negative breast cancer (TNBC). This therapy boosts the body’s immune system to identify and destroy cancer cells, offering a more targeted approach than traditional methods like chemotherapy or radiation. Understanding immunotherapy’s potential is crucial for patients, as it represents a promising option for cases that resist other treatments. For example, drugs like pembrolizumab (Keytruda) have shown improved survival rates in patients with high PD-L1 expression.
Despite its potential, immunotherapy can lead to immune-related side effects, including issues with the skin, lungs, and organs, which must be carefully monitored. Current clinical trials continue to explore its effectiveness, with some showing durable responses in patients who previously had limited options. This article will discuss the types of immunotherapy available, review success rates, and explore the side effects and risks involved, supported by recent research.
Immunotherapy is a promising cancer treatment that harnesses the body’s immune system to target and destroy cancer cells. In breast cancer, especially aggressive forms like triple-negative breast cancer (TNBC), immunotherapy is increasingly being used when conventional treatments such as chemotherapy are less effective. The primary mechanism of action involves immune checkpoint inhibitors that block proteins like PD-1 and PD-L1, which normally suppress the immune response. By inhibiting these proteins, immunotherapy “releases the brakes” on the immune system, allowing T-cells to more effectively identify and attack cancer cells.
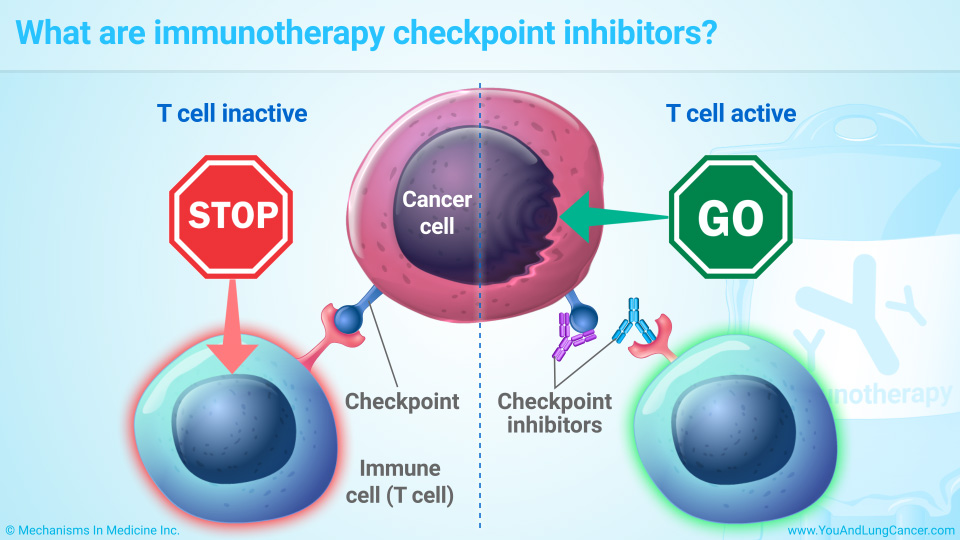
Additionally, other forms of immunotherapy, such as cancer vaccines and CAR T-cell therapy, are being researched to further enhance the immune response. These therapies help either stimulate the immune system or modify immune cells to specifically target cancer.
Immunotherapy includes checkpoint inhibitors that boost T-cell activity, monoclonal antibodies that target cancer proteins, cancer vaccines that stimulate the immune system, and CAR T-cell therapy, which modifies T-cells to attack cancer directly.
Checkpoint Inhibitors
Drugs like pembrolizumab (Keytruda) and nivolumab (Opdivo) are immune checkpoint inhibitors that work by blocking the interaction between PD-1 on T-cells and PD-L1 on cancer cells. This mechanism enhances the immune system’s ability to detect and destroy cancer cells by “releasing the brakes” on T-cells. By inhibiting these proteins, the immune system is better able to recognize and attack cancerous tissues.
In the context of breast cancer, particularly triple-negative breast cancer (TNBC), pembrolizumab has demonstrated significant clinical efficacy. According to a study by Schmid et al., published in the New England Journal of Medicine in 2021, the KEYNOTE-355 trial showed that pembrolizumab, when added to chemotherapy, improved progression-free survival (PFS) in patients with PD-L1-positive TNBC, reducing the risk of disease progression by 35%.
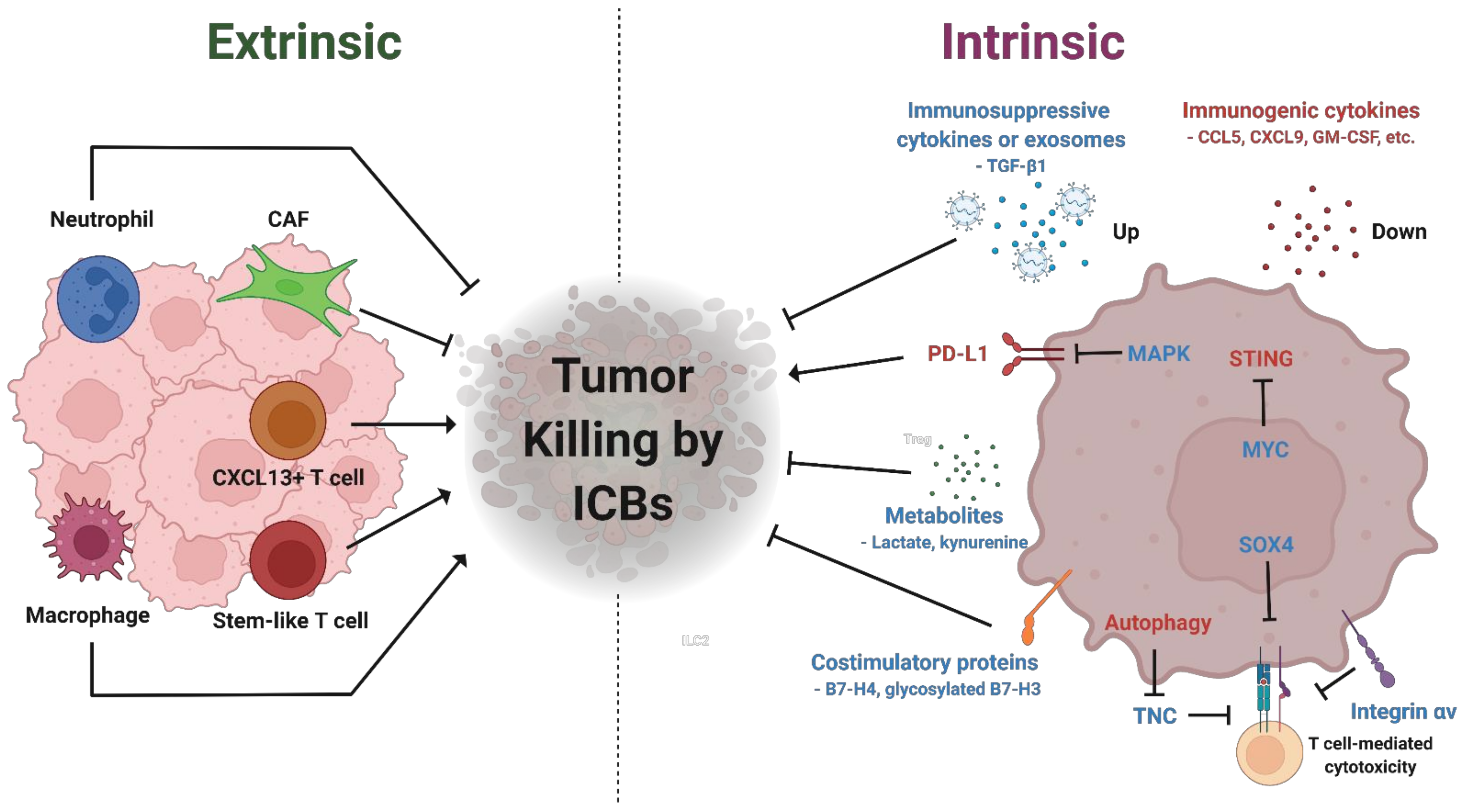
Source: Kim et al, Biomedicine, 2022
This trial highlights the potential of immunotherapy in managing aggressive cancer subtypes like TNBC, offering better outcomes for patients who typically have fewer treatment options
Monoclonal Antibodies
Trastuzumab (Herceptin) is a monoclonal antibody used to treat HER2-positive breast cancer, which overexpresses the HER2 protein, driving tumor growth. Trastuzumab binds to HER2 receptors, blocking growth signals and triggering the immune system to destroy cancer cells. According to Slamon et al. (2011), trastuzumab improves overall survival by 37% in early-stage HER2-positive breast cancer. Combined with chemotherapy, it reduces recurrence rates by 50%. In advanced cases, combining trastuzumab with pertuzumab (dual HER2 blockade) significantly extends survival, as shown in the CLEOPATRA trial, where patients lived 16 months longer on average compared to trastuzumab alone.
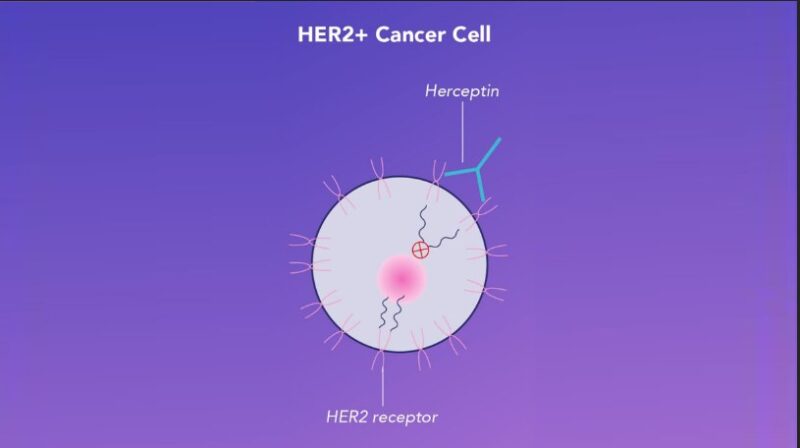
Cancer Vaccines
Cancer vaccines are a growing form of immunotherapy that aim to stimulate the immune system to recognize and attack cancer cells. Preventive vaccines, like the HPV vaccine, prevent cancers from infections, while therapeutic vaccines, like Sipuleucel-T (Provenge), treat existing cancers.
Provenge: In the IMPACT trial, Provenge extended median overall survival by 4.1 months in metastatic castration-resistant prostate cancer and improved 3-year survival to 31.7% compared to 21.7% for placebo.
Breast Cancer Vaccines: Research on HER2-targeted vaccines and mRNA vaccines is ongoing, showing early promise in reducing recurrence in HER2-positive cases. However, no vaccines for breast cancer are currently FDA-approved, and more clinical trials are needed.
CAR T-Cell Therapy
CAR T-cell therapy is an innovative immunotherapy that modifies a patient’s T-cells to attack cancer cells. While it has been successful in blood cancers like leukemia, its use in breast cancer remains experimental. Early trials, such as a 2017 study by Feng et al., showed partial tumor reductions in HER2-positive breast cancer, though responses varied.
A major challenge is the tumor microenvironment, which hinders CAR T-cells from effectively reaching cancer cells. Combination therapies, like using checkpoint inhibitors, are being studied to enhance CAR T-cell efficacy.
Though promising, CAR T-cell therapy in breast cancer faces obstacles like antigen escape and side effects such as cytokine release syndrome (CRS). Further research is needed to improve its effectiveness and safety.
In Triple-Negative Breast Cancer (TNBC), checkpoint inhibitors like pembrolizumab (Keytruda) have shown significant effectiveness, particularly when combined with chemotherapy. The KEYNOTE-355 trial demonstrated an improvement in progression-free survival (PFS) from 5.6 months to 9.7 months in patients with PD-L1-positive metastatic TNBC, with an overall response rate of approximately 53%. However, the effectiveness of this combination therapy is primarily observed in PD-L1-positive patients, highlighting the importance of biomarker testing in treatment planning.
For HER2-positive breast cancer, trastuzumab (Herceptin), a monoclonal antibody, has revolutionized treatment. When combined with chemotherapy in early-stage HER2-positive patients, it has led to 5-year survival rates exceeding 90% and reduced the risk of recurrence by 50%, significantly improving both disease-free and overall survival. This combination therapy is now considered the standard of care, offering substantial long-term benefits for patients with HER2-positive tumors.
Meanwhile, CAR T-cell therapy is still in the experimental phase for breast cancer but has shown potential in treating HER2-positive tumors. Early trials have reported partial tumor responses, though results are variable and inconsistent. The primary challenge with CAR T-cell therapy in breast cancer lies in the tumor microenvironment and antigen escape, which limit the therapy’s effectiveness in solid tumors like breast cancer.
Immunotherapy, especially pembrolizumab (Keytruda), has shown promise in treating triple-negative breast cancer (TNBC). In the KEYNOTE-355 trial, combining pembrolizumab with chemotherapy improved progression-free survival from 5.6 to 9.7 months and increased the overall response rate to 53% in PD-L1-positive metastatic TNBC patients. Similarly, the KEYNOTE-522 trial showed a pathological complete response (pCR) rate of 64.8% in early-stage TNBC with pembrolizumab, compared to 51.2% in the chemotherapy-only group, highlighting its effectiveness in both early and advanced TNBC
Immunotherapy research in HER2-positive breast cancer is advancing, complementing established targeted therapies like trastuzumab, which has shown a 10-year overall survival rate of 84% in the HERA trial. While trastuzumab combined with chemotherapy has reduced recurrence risk by 50% in early-stage patients, researchers are exploring immunotherapeutic approaches to further improve outcomes. The PANACEA trial investigated pembrolizumab with trastuzumab in pretreated HER2-positive metastatic breast cancer, showing an overall response rate of 15% in PD-L1-positive patients.
Novel agents like zanidatamab (ZW25), a bispecific antibody targeting two HER2 epitopes, and trastuzumab deruxtecan (T-DXd), an antibody-drug conjugate, are showing promise in clinical trials. The DESTINY-Breast01 trial reported a 60.9% objective response rate with T-DXd in heavily pretreated HER2-positive metastatic breast cancer patients. Ongoing research focuses on overcoming resistance, developing predictive biomarkers, optimizing combination therapies, and exploring personalized immunotherapeutic approaches for HER2-positive breast cancer patients.
Read More about how Targeted Therapydiffers from Immunotherapy: Article by OncoDaily

Immunotherapy for breast cancer can lead to side effects ranging from fatigue and skin rashes to more severe issues like diarrhea and autoimmune reactions. Fatigue is persistent and may require lifestyle changes, while skin rashes, especially with drugs like pembrolizumab, are often treated with topical creams or corticosteroids. Diarrhea may signal colitis, a serious condition needing urgent care. Severe autoimmune reactions, such as hepatitis or pneumonitis, may require immunosuppressive treatments. Regular monitoring and prompt intervention are essential for managing these effects and preserving the patient’s quality of life
• Fatigue: Affects up to 80% of chemotherapy patients and 40% of those on immunotherapy. It is often described as persistent exhaustion that doesn’t improve with rest, resulting from the immune response and damage to healthy cells.
• Nausea and Vomiting: Experienced by 70-80% of chemotherapy patients due to gut irritation and brain receptor activation. Less common in immunotherapy, nausea is managed with antiemetics like ondansetron.
• Skin Toxicities: Impact 40% of immunotherapy patients, especially those on checkpoint inhibitors. Symptoms include rashes and itching, often managed with corticosteroids.
Management: Supportive care such as antiemetics, corticosteroids, and fatigue management techniques are crucial to improving patient quality of life and maintaining treatment continuity.

Autoimmune reactions (irAEs) and infusion reactions are serious side effects of immunotherapy that require careful management. Autoimmune reactions occur when the immune system mistakenly attacks healthy organs. Common examples include colitis, which causes severe diarrhea and abdominal pain, and pneumonitis, leading to difficulty breathing and persistent cough. For instance, a patient on pembrolizumab (Keytruda) developed pneumonitis, requiring treatment with high-dose corticosteroids to reduce inflammation. Infusion reactions can happen during or shortly after treatment and range from mild symptoms like fever and chills to severe, life-threatening conditions such as anaphylaxis.
Autoimmune reactions are typically managed with corticosteroids or stronger immunosuppressive drugs like infliximab, while infusion reactions are treated with antihistamines and close monitoring during treatment. Around 10-20% of patients on immunotherapy experience irAEs, and 1-3% may develop serious infusion reactions. Close monitoring and early intervention are essential for managing these side effects and ensuring the continuation of immunotherapy
Immunotherapy for stage 4 breast cancer focuses on prolonging life and managing symptoms using chemotherapy, immunotherapy, targeted therapies like trastuzumab for HER2-positive cancers, and hormonal treatments for hormone receptor-positive cases. Immunotherapy has shown promise in triple-negative breast cancer (TNBC), improving survival outcomes.
The KEYNOTE-355 study (Cortes et al., 2020) demonstrated that adding pembrolizumab (Keytruda) to chemotherapy improved both overall survival and progression-free survival in patients with PD-L1-positive metastatic TNBC, significantly increasing median survival. Similarly, the IMpassion130 trial (Schmid et al., 2018) showed that atezolizumab (Tecentriq) combined with chemotherapy extended progression-free and overall survival in PD-L1-positive TNBC.
These advances have led to median survival times of 24 to 36-50 months, representing significant progress in managing stage 4 breast cancer.
Combination immunotherapies, such as nivolumab (PD-1 inhibitor) and ipilimumab (CTLA-4 inhibitor), aim to enhance the immune response by targeting two immune checkpoints. While successful in cancers like melanoma and lung cancer, ongoing studies are evaluating their potential in triple-negative breast cancer (TNBC), particularly for patients with high tumor mutational burden (TMB).
Clinical trials have reported response rates of 20-30% in these patients, with improvements in progression-free survival. However, this combination increases the risk of immune-related adverse events (irAEs), such as colitis, pneumonitis, and hepatitis, requiring close monitoring and, in severe cases, treatment with corticosteroids or immunosuppressants.
Though promising for patients with limited options in advanced breast cancer, these therapies are still investigational, with more research needed to assess long-term benefits and risks.
Single-agent immunotherapies, such as pembrolizumab (Keytruda) and atezolizumab (Tecentriq), have shown promise in treating stage 4 triple-negative breast cancer (TNBC), particularly when combined with chemotherapy. In the KEYNOTE-355 trial, pembrolizumab combined with chemotherapy improved progression-free survival (PFS) and overall survival in patients with metastatic TNBC, regardless of PD-L1 status. Similarly, atezolizumab, though used primarily for PD-L1-positive patients, has shown moderate success with improved outcomes when combined with chemotherapy, as demonstrated in the IMpassion130 trial.
For HER2-positive breast cancer, trastuzumab remains the standard, while newer drugs like Trastuzumab Deruxtecan (T-DXd) are expanding treatment options for HER2-low and HER2-ultra-low tumors. The DESTINY-Breast03 trial showed that T-DXd significantly improved survival in HER2-positive patients, and ongoing research is exploring its application to HER2-low cancers, offering new options for those with minimal HER2 expression.
These advancements continue to reshape the treatment landscape for both TNBC and HER2-positive breast cancers, providing new hope for patients with limited therapeutic options.
Immunotherapy for breast cancer is typically administered via intravenous (IV) infusions in a hospital or clinic, with sessions lasting 30 minutes to an hour. Drugs like pembrolizumab and atezolizumab are usually given every 2 to 3 weeks, depending on the regimen. Pembrolizumab is often dosed every 3 weeks, and in some cases, every 6 weeks. Atezolizumab is generally given every 3 weeks, often with chemotherapy.
Treatment duration varies but can last up to 2 years or more, depending on the patient’s response and tolerance. For HER2-positive breast cancer, trastuzumab can be administered via subcutaneous injections, offering quicker treatment and flexibility, typically on a 3-week schedule.
Regular monitoring helps adjust treatment schedules and manage side effects to optimize outcomes.
During immunotherapy for breast cancer, patients undergo a process that starts with an initial consultation with their oncologist. Here, they discuss their specific cancer type, potential treatment options. Once approved, the treatment typically involves IV infusions, which can last from 30 minutes to several hours per session.
As the treatment begins, patients are closely monitored for immediate side effects, such as infusion reactions like fever or allergic symptoms. Common side effects of immunotherapy include fatigue, skin rashes, and nausea. Some patients might experience more severe effects, such as autoimmune reactions, which can target healthy organs like the colon or liver. Managing these side effects often involves medications like corticosteroids or anti-nausea drugs.
Throughout the treatment, patients’ progress is monitored with imaging tests (CT or MRI) and regular blood work to evaluate how well the treatment is working and to adjust the treatment plan if needed. Once active treatment ends, follow-up care becomes essential, involving regular check-ups to detect any recurrence or long-term side effects. Emotional and psychological support also plays a vital role in the overall treatment experience, helping patients cope with stress and uncertainty
Initial responses to immunotherapy, such as tumor shrinkage or stabilization, typically appear within 6 to 12 weeks after starting treatment, though timelines vary based on the cancer type, treatment, and individual response. Some patients may experience pseudoprogression, where tumors initially appear larger before shrinking due to immune cell activity.
Most patients show a partial or complete response within 3 to 6 months, while others may see a delayed response after several months. Some may achieve stable disease, where the cancer doesn’t grow but also doesn’t shrink significantly.
In cancers like melanoma and non-small cell lung cancer (NSCLC), long-term benefits have been observed, with some patients experiencing remission lasting several years. Regular imaging and blood tests are critical to monitor progress and adjust the treatment plan, as immunotherapy responses often take longer than traditional treatments, requiring patience and careful monitoring.
Checkpoint inhibitors, like pembrolizumab (Keytruda) or nivolumab (Opdivo), can cost between $100,000 to $150,000 per year, depending on dosage and duration, while CAR T-cell therapy can exceed $373,000 for a single treatment. Although most private insurers, Medicare, and Medicaid cover FDA-approved immunotherapies, patients may still face significant out-of-pocket costs, including co-pays and deductibles.
To help manage these expenses, pharmaceutical assistance programs (e.g., Merck for Keytruda, Bristol-Myers Squibb for Opdivo) and non-profit organizations like the CancerCare Foundation or Leukemia & Lymphoma Society provide financial aid. Additionally, clinical trials offer access to experimental treatments at no cost, though eligibility is required.
Patients should explore financial counseling and payment plans at cancer centers to mitigate the financial burden and ensure continued access to treatment. Understanding and utilizing these resources is crucial to managing the cost of immunotherapy
Immunotherapy, especially in combination with chemotherapy, has shown encouraging results for triple-negative breast cancer (TNBC). According to the KEYNOTE-355 trial, adding pembrolizumab to chemotherapy significantly improved progression-free survival (PFS) to 9.7 months for patients with PD-L1-positive TNBC, compared to chemotherapy alone.
For early-stage cancers, surgery remains the most effective treatment option, offering 5-year survival rates exceeding 90%. Chemotherapy, while effective, often comes with side effects such as fatigue and immunosuppression.
Read More about how Chemotherapy differs from Immunotherapy: Article by OncoDaily
Immunotherapy can provide durable responses and long-term remission for some breast cancer patients, particularly those with triple-negative breast cancer treated with checkpoint inhibitors like pembrolizumab. These treatments often lead to extended periods without cancer progression, improving survival rates and quality of life. However, they can also cause chronic immune-related adverse events (irAEs), such as hypothyroidism or colitis, which may require lifelong management. Other potential long-term effects include persistent fatigue and complications involving the endocrine, pulmonary, or cardiovascular systems, necessitating ongoing medical monitoring.
Immunotherapy has shown significant progress in treating triple-negative breast cancer (TNBC), particularly with pembrolizumab (Keytruda) combined with chemotherapy, as demonstrated in the KEYNOTE-355 trial. This trial, published by Schmid et al. in The Lancet (2020), showed improved progression-free survival (PFS) and overall survival in TNBC patients, regardless of PD-L1 expression.
Recent research suggests that TNBC patients without high PD-L1 expression may also benefit from immunotherapy. Emerging biomarkers such as tumor-infiltrating lymphocytes (TILs) and tumor mutational burden (TMB) are being investigated to further expand the applicability of immunotherapy in TNBC. Ongoing clinical trials are exploring the combination of checkpoint inhibitors with targeted therapies to improve outcomes for both PD-L1-positive and PD-L1-negative patients.
According to Schmid et al. (2020) and ongoing research published in Current Oncology Reports (2023), new biomarkers could help extend the benefits of immunotherapy beyond PD-L1 expression alone, broadening treatment options for more TNBC patients.
Research on immunotherapy for breast cancer is rapidly advancing, with numerous clinical trials exploring new drugs and combination treatments. A key focus is on combining checkpoint inhibitors, like pembrolizumab (Keytruda) and atezolizumab (Tecentriq), with standard therapies such as chemotherapy. Trials like KEYNOTE-355 and IMpassion130 have shown encouraging results, particularly in triple-negative breast cancer (TNBC), where these combinations have improved progression-free survival in PD-L1-positive patients.
New immunotherapy drugs, such as bispecific antibodies and TIGIT inhibitors, are also being investigated to overcome resistance to existing treatments. Another promising area is the development of cancer vaccines, designed to stimulate the immune system to target breast cancer cells, including HER2-directed vaccines to prevent recurrence in HER2-positive patients.
Additionally, CAR T-cell therapy, which has been successful in treating blood cancers, is now being adapted for breast cancer. Clinical trials are testing its effectiveness against HER2 and other antigens specific to breast cancer.
Finally, research is ongoing to address immunotherapy resistance. Combination strategies, including epigenetic drugs and CDK4/6 inhibitors, are being explored to enhance the effectiveness of immunotherapy across various breast cancer subtypes. These efforts aim to expand the role of immunotherapy and improve outcomes for breast cancer patients.
Read More about Breast Cancer Research Day:Where are we in 2024: Article by Oncodaily
During immunotherapy, supporting the immune system through lifestyle changes, dietary adjustments and supplements can be beneficial. Here are some key recommendations:
During immunotherapy, making thoughtful lifestyle changes and dietary adjustments can support the immune system. Regular moderate exercise, such as walking or yoga, can boost immune function and help reduce fatigue. Managing stress through techniques like mindfulness or meditation is also important for maintaining immune health. Adequate sleep is essential, as rest plays a crucial role in the recovery and functioning of the immune system.
Dietary changes, such as consuming a balanced diet rich in fruits, vegetables, lean proteins, and whole grains, provide essential nutrients needed for immune support. Foods high in antioxidants, like berries and spinach, can help combat inflammation, while staying hydrated supports overall health and detoxification.
Some supplements, including vitamin D, zinc, and omega-3 fatty acids, may also help strengthen the immune response. However, it’s important to consult your doctor before starting any supplements to ensure they are safe and won’t interfere with your treatment.
These adjustments can help enhance the body’s natural defenses and improve overall well-being during immunotherapy.
What Is Immunotherapy and How Does It Differ From Traditional Cancer Treatments?
Immunotherapy works by boosting the body’s immune system to target and destroy cancer cells, unlike traditional treatments like chemotherapy and radiation, which directly attack cancer cells but can also harm healthy cells. Immunotherapy is often more targeted, focusing on enabling the immune system to fight cancer with fewer side effects.
Can Immunotherapy Be Used for All Types of Breast Cancer?
Not all types of breast cancer respond well to immunotherapy. It is most effective for certain subtypes, such as triple-negative breast cancer (TNBC) and HER2-positive breast cancer, especially in cases where the cancer has spread or recurred. Ongoing research aims to expand its use for other breast cancer types.
How Does Immunotherapy Improve Survival Rates for Breast Cancer Patients?
Immunotherapy, particularly with drugs like pembrolizumab, has been shown to improve progression-free survival in patients with triple-negative breast cancer. In clinical trials, patients with PD-L1-positive tumors have experienced better outcomes, including longer survival rates compared to traditional treatments alone.
What Are the Potential Side Effects of Immunotherapy?
Common side effects of immunotherapy include fatigue, skin rashes, and diarrhea. More serious side effects, such as autoimmune reactions, can occur when the immune system attacks healthy tissues, causing conditions like colitis, hepatitis, or pneumonitis. Monitoring is essential to manage these risks effectively.
Is Immunotherapy Effective for Stage 4 Breast Cancer?
For stage 4 breast cancer, immunotherapy is often used in combination with chemotherapy to help manage the disease and prolong life. While it may not cure advanced cancer, it can help shrink tumors, slow disease progression, and extend survival in patients, especially those with triple-negative or HER2-positive breast cancer.
Can Immunotherapy Be Combined With Other Treatments?
Yes, immunotherapy is often combined with chemotherapy, targeted therapies, or even radiation to enhance its effectiveness. For example, combining pembrolizumab with chemotherapy has shown to improve outcomes in triple-negative breast cancer. Ongoing research is also investigating the benefits of combining it with new immunotherapy drugs.
How Long Does Immunotherapy Treatment Typically Last?
Immunotherapy treatments for breast cancer are generally administered for up to 2 years, depending on how well the patient responds and tolerates the therapy. Some patients may continue treatment longer if it remains effective, while others might discontinue due to side effects or completion of a prescribed regimen.
Can Patients With HER2-Positive Breast Cancer Benefit From Immunotherapy?
HER2-positive breast cancer patients primarily benefit from targeted therapies like trastuzumab (Herceptin), but ongoing studies are exploring the potential of combining these treatments with immunotherapy. Early research suggests that this combination may further improve outcomes for advanced HER2-positive cases.
Are There Clinical Trials for Immunotherapy for Breast Cancer?
Yes, numerous clinical trials are currently investigating new immunotherapy drugs, combinations with existing therapies, and their effectiveness in different breast cancer subtypes. Patients interested in joining a trial should speak with their oncologist to see if they are eligible based on their cancer type and stage.
How Can I Support My Immune System During Immunotherapy?
To support your immune system during immunotherapy, maintain a healthy diet, stay hydrated, get regular exercise, manage stress, and ensure adequate sleep. Avoid smoking and alcohol, and consult your healthcare provider before taking any supplements to ensure they complement your treatment plan.
The article is written by Toma Oganezova, MD, and is taken from OncoDaily.
About IMMONC
Immune Oncology Research Institute (IMMONC) is dedicated to advancing research aimed at preventing, treating, and ultimately curing cancer while making these innovations accessible to those who need them. If you're interested in joining our team, please feel free to contact us at [email protected] or at +374-41 310-048.