
Blood cancers, also known as hematologic cancers, affect the production and function of blood cells. These cancers typically begin in the bone marrow, where blood cells are produced. The three main types of blood cancer are leukemia, lymphoma, and multiple myeloma. Blood cancers account for about 10% of all diagnosed cancers in the United States each year and are responsible for approximately 3% of all cancer-related deaths. The prevalence of blood cancers has been increasing, but advancements in treatment have significantly improved survival rates.
Immunotherapy is a type of cancer treatment that modulates the body’s immune system to combat cancer. Unlike traditional treatments such as chemotherapy and radiation, which directly target cancer cells, immunotherapy works by enhancing or modifying the immune system’s natural ability to detect and destroy cancer cells. This approach can be particularly effective because it can provide a more targeted attack on cancer cells while potentially sparing healthy cells.
Immunotherapy has revolutionized the treatment of blood cancers, offering new hope for patients with various hematologic malignancies. It can be used alone or in combination with other treatments such as chemotherapy, radiation, and stem cell transplantation. The effectiveness of immunotherapy in treating blood cancers has been remarkable, with some patients achieving long-lasting remissions and improved survival rates. However, it is important to note that the success of immunotherapy can vary depending on the specific type of blood cancer, the stage of the disease, and the individual patient’s response. Immunotherapy is used in various scenarios for treating blood cancers, including:
• First-Line Treatment: For certain types of blood cancers, immunotherapy can be used as the initial treatment, either alone or in combination with other therapies.
• Combination Therapy: Immunotherapy is often combined with chemotherapy, radiation, or steroids to enhance the overall treatment response.
• Rescue Therapy: For patients whose cancer has not responded to other treatments or has relapsed, immunotherapy can be used as a rescue therapy.
• Pre-Stem Cell Transplant: In some cases, immunotherapy is used before a stem cell transplant to reduce the cancer burden and improve the chances of a successful transplant.
• Maintenance Therapy: After initial treatment, immunotherapy can be used as maintenance therapy to prevent relapse and prolong remission.
Leukemia is a type of blood cancer that affects the body’s ability to produce normal blood cells. It begins in the bone marrow, where blood cells are produced, and results in an overproduction of abnormal white blood cells. These abnormal cells crowd out healthy blood cells, leading to various complications and symptoms. Leukemia can be classified into different types based on the specific blood cell affected and the rate of progression.
Immunotherapy has shown promising results in treating various types of leukemia, including:
• Acute Lymphoblastic Leukemia (ALL)
• Acute Myeloid Leukemia (AML)
• Chronic Lymphocytic Leukemia (CLL)
• Chronic Myeloid Leukemia (CML)
Several immunotherapy approaches have been developed and approved for the treatment of leukemia, including:
• Monoclonal Antibodies: These lab-made proteins can target specific antigens on leukemia cells, marking them for destruction by the immune system. Examples include rituximab, ofatumumab, and gemtuzumab ozogamicin.
• Chimeric Antigen Receptor (CAR) T-Cell Therapy: In this approach, a patient’s T-cells are genetically engineered to produce receptors that can recognize and attack leukemia cells. Approved CAR T-cell therapies for leukemia include tisagenlecleucel and brexucabtagene autoleucel.
• Immune Checkpoint Inhibitors: These drugs help the immune system recognize and attack leukemia cells by blocking the inhibitory signals that cancer cells use to evade immune detection. Examples include nivolumab and pembrolizumab.
• Cytokines: These are proteins that can stimulate the immune system to fight leukemia cells more effectively. Examples include interleukin-2 (IL-2) and interferon-alpha.
Immunotherapy has shown promising results in treating various types of leukemia, particularly in cases where other treatments have failed or relapsed. For example, in clinical trials for CAR T-cell therapy in relapsed or refractory ALL, complete remission rates ranging from 60% to 90% have been reported. Monoclonal antibodies, such as rituximab, have also demonstrated improved survival rates and remission durations when used in combination with chemotherapy for certain types of leukemia.
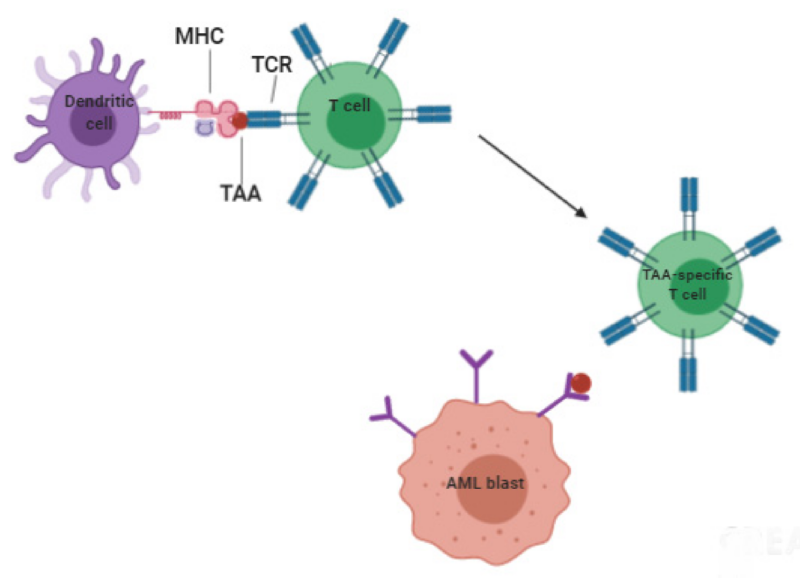
A schematic representation of T cell responses to AML tumor-associated antigens shows dendritic cells presenting MHC molecules, loaded with AML antigen peptides, to the T cell receptors on T lymphocytes. This interaction triggers the activation and proliferation of AML-specific T cells. Among these, cytotoxic T lymphocytes (CTLs) can identify and destroy AML cells. This image is taken from the article by Aureli et al. (2021).
Lymphoma is a type of blood cancer that originates in the lymphatic system, which is part of the body’s immune system. It involves the uncontrolled growth of lymphocytes, a type of white blood cell. Lymphoma is broadly categorized into Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL), with NHL being more common. Both types can affect lymph nodes and other organs, leading to symptoms such as swollen lymph nodes, fever, night sweats, and weight loss. Immunotherapy has become a crucial treatment option for lymphoma, offering targeted approaches to enhance the immune system’s ability to fight cancer cells.
Immunotherapy is used to treat various types of lymphoma, including:
Hodgkin Lymphoma
Non-Hodgkin Lymphoma:
• Diffuse Large B-cell lymphoma (DLBCL)
• Follicular Lymphoma (FL)
• Mantle Cell Lymphoma (MCL)
• T-Cell Lymphomas
Monoclonal Antibodies
• Rituximab (Rituxan): Targets CD20 on B-cells. It is used for various B-cell lymphomas, including DLBCL and FL. In combination with chemotherapy, rituximab has improved overall survival rates in patients with DLBCL and FL. Long-term follow-up studies have shown that rituximab maintenance therapy can prolong remission in FL patients.
• Obinutuzumab (Gazyva): Another anti-CD20 antibody, used for FL and CLL. It has shown superior efficacy compared to rituximab in some studies, particularly in the first-line treatment of FL.
• Brentuximab Vedotin (Adcetris): Targets CD30 and is used for HL and some T-cell lymphomas. Clinical trials have demonstrated high response rates, with many patients achieving complete remission.
Immune Checkpoint Inhibitors
• Nivolumab (Opdivo): Approved for relapsed or refractory HL. Clinical trials have shown that about two-thirds of patients respond to nivolumab, with many experiencing durable remissions.
• Pembrolizumab (Keytruda): Used for relapsed or refractory HL and some types of NHL. It has shown promising results, with significant response rates and manageable side effects.
CAR T-Cell Therapy
• Axicabtagene Ciloleucel (Yescarta): Approved for DLBCL and other aggressive B-cell lymphomas.
• Tisagenlecleucel (Kymriah): Used for DLBCL and FL. Axicabtagene ciloleucel and tisagenlecleucel have shown complete response rates of around 40-50% in patients with refractory DLBCL, with many patients experiencing long-term remissions.
• Lisocabtagene Maraleucel (Breyanzi): Approved for DLBCL and other B-cell lymphomas. It has demonstrated significant efficacy, with a substantial proportion of patients achieving complete responses.
Bispecific Antibodies: These antibodies can bind to two different targets simultaneously, typically bringing T-cells into close proximity with cancer cells to promote their destruction.
• Blinatumomab (Blincyto): Targets CD19 on B-cells and CD3 on T-cells. It is used for B-cell precursor acute lymphoblastic leukemia (ALL) and has shown high response rates in clinical trials.
• Mosunetuzumab: Targets CD20 on B-cells and CD3 on T-cells. It has shown promising results in clinical trials for R/R B-cell NHL, with significant response rates.
The trial presented in this video concluded that replacing rituximab with obinutuzumab in patients with untreated follicular lymphoma extended progression-free survival but led to more high-grade adverse events.
Multiple myeloma (MM) is a type of blood cancer that originates in the plasma cells, a form of white blood cell responsible for producing antibodies. It is characterized by the uncontrolled proliferation of these cells within the bone marrow, leading to various complications such as bone damage, anemia, renal failure, and immune system impairment. Despite significant advancements in treatment, MM remains incurable, with a high rate of relapse. Immunotherapy has emerged as a promising approach in MM treatment, aiming to harness the patient’s immune system to recognize and combat cancer cells more effectively.
Monoclonal Antibodies
• Daratumumab (Darzalex) and Isatuximab (Sarclisa) target the CD38 protein on MM cells. Daratumumab has shown to improve survival rates when used in combination with other MM treatments.
• Elotuzumab (Empliciti) targets SLAMF7, another surface protein on MM cells, and is used in combination with other therapies to treat patients who have received one to three prior medications.
Bispecific T-Cell Engagers (BiTEs)
• Teclistamab (Tecvayli), a BCMA-directed BiTE, has been approved for patients with relapsed or refractory MM who have received at least four prior lines of therapy, showing an overall response rate of 61.8% in clinical trials.
• Talquetamab (Talvey), another BiTE targeting GPRC5D, a protein highly expressed on MM cells, has shown promising results in clinical trials, with a significant proportion of patients responding to treatment.
CAR T-Cell Therapy
• Idecabtagene Vicleucel (Abecma) and Ciltacabtagene Autoleucel (Carvykti) are BCMA-targeted CAR T-cell therapies approved for patients with relapsed or refractory MM. These therapies have demonstrated high efficacy, with a significant number of patients achieving complete remission. The introduction of CAR T-cell therapy has significantly improved outcomes for patients with advanced MM, offering a potential for long-term remission in some cases.
While immunotherapy can be highly effective, it is also associated with various side effects. These side effects can range from mild to severe and are often different from those associated with traditional cancer treatments like chemotherapy and radiation. Understanding these side effects is crucial for managing them effectively and ensuring the best possible outcomes for patients.
Common Side Effects
• Fatigue: One of the most common side effects of immunotherapy is fatigue, which can be both physically and mentally exhausting. This type of fatigue is different from everyday tiredness and can significantly impact a patient’s quality of life.
• Skin Reactions: Immunotherapy can cause various skin issues, including rashes, dryness, itching, and sensitivity to sunlight. In some cases, patients may experience blistering or cracking of the skin.
• Gastrointestinal Issues: Diarrhea, colitis (inflammation of the colon), nausea, vomiting, and abdominal pain are common gastrointestinal side effects. These can range from mild to severe and may require medical intervention.
• Flu-like Symptoms: Patients may experience symptoms similar to the flu, such as fever, chills, and muscle aches. These symptoms are usually temporary but can be quite uncomfortable.
• Endocrine Disorders: Immunotherapy can affect the endocrine system, leading to conditions such as hypothyroidism, hyperthyroidism, and adrenal insufficiency. These conditions may require long-term hormone replacement therapy.
Severe Side Effects
• Immune-Related Adverse Events (irAEs): These occur when the immune system attacks healthy tissues and organs. irAEs can affect any part of the body but most commonly impact the skin, colon, endocrine organs, liver, joints, and lungs. Severe irAEs can be life-threatening and require prompt medical attention.
• Neurotoxicity: CAR T-cell therapy, a type of immunotherapy, can cause neurotoxic effects such as confusion, seizures, and difficulty speaking or walking. Most cases of neurotoxicity are resolved with close monitoring and appropriate treatment.
• Cardiac and Renal Impairment: Serious toxicities can also include cardiac issues, such as myocarditis, and renal impairment. These side effects are less common but can be severe and require immediate medical intervention.
Management of Side Effects
• Early Detection and Monitoring: Early detection of side effects is crucial for effective management. Regular monitoring and prompt assessment of symptoms can help mitigate severe complications.
• Steroid Therapy: For higher-grade toxicities, steroids are often used to reduce inflammation and manage symptoms. The dosage and duration of steroid therapy depend on the severity of the side effects.
• Supportive Care: Managing side effects also involves supportive care measures such as hydration, nutritional support, and pain management. Patients may also benefit from psychological support to cope with the emotional and mental stress of treatment.
• Specialist Consultation: In cases of severe side effects, consultation with specialists such as endocrinologists, dermatologists, and gastroenterologists may be necessary to provide targeted treatment and care.
Immunotherapy as a Turning Point in the Treatment of Acute Myeloid Leukemia – Cancers
Obinutuzumab for the First-Line Treatment of Follicular Lymphoma – NEJM
Immunotherapy approaches for hematological cancers – iScience
Immunotherapies in Non-Hodgkin’s Lymphoma – Cancers
New developments in immunotherapy for lymphoma – Cancer Biology and Medicine
Introduction to “Immunotherapies for Multiple Myeloma” – Pharmaceuticals
Side Effects of Immunotherapy – American Society of Clinical Oncology
The article is taken from OncoDaily
About IMMONC
Immune Oncology Research Institute (IMMONC) is dedicated to advancing research aimed at preventing, treating, and ultimately curing cancer while making these innovations accessible to those who need them. If you're interested in joining our team, please feel free to contact us at [email protected] or at +374-41 310-048.