
Immunotherapy is a revolutionary approach to cancer treatment that utilizes the power of the body’s own immune system to fight against cancer cells. In the case of colorectal cancer, this approach has shown remarkable success in a subset of patients whose tumors exhibit specific genetic characteristics. The immune system is designed to recognize and eliminate abnormal or foreign cells, but cancer cells can sometimes evade this surveillance mechanism. Immunotherapy aims to restore and enhance the immune system’s ability to detect and destroy cancer cells.
The most commonly used immunotherapy for colorectal cancer targets specific proteins called immune checkpoints, which are responsible for regulating the immune response. Cancer cells can exploit these checkpoints to suppress the immune system’s ability to recognize and attack them. Immune checkpoint inhibitors, such as pembrolizumab (Keytruda) and nivolumab (Opdivo), work by blocking these checkpoint proteins, thereby restoring the immune system’s full potential to target and eliminate cancer cells. This approach has proven particularly effective in colorectal cancers with deficient mismatch repair (dMMR) or high microsatellite instability (MSI-H), which are genetic abnormalities that lead to an accumulation of mutations in cancer cells, making them more visible to the immune system.
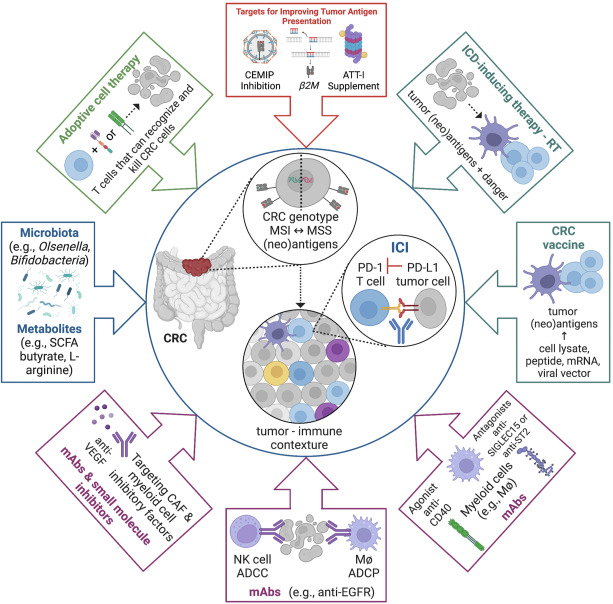
The image illustrates the various investigated pathways for colorectal cancer (CRC) immunotherapy. Immune checkpoint inhibitors (ICIs) are at the center of CRC immunotherapy, while several strategies are combined with ICIs to improve therapy outcomes.
ADCC – antibody-dependent cellular cytotoxicity; ADCP – antibody-dependent cellular phagocytosis; EGFR – epidermal growth factor receptor; M4 – macrophage; VEGF – vascular endothelial growth factor.
Image source: Cornista et al. (2023).
Several immunotherapy drugs have been approved for the treatment of colorectal cancer, primarily targeting the immune checkpoint proteins PD-1 and CTLA-4. These drugs can be used alone or in combination with other therapies, depending on the specific characteristics of the patient’s cancer.
PD-1 Inhibitors
• Pembrolizumab (Keytruda): Pembrolizumab is a monoclonal antibody that targets the PD-1 protein on immune cells, preventing cancer cells from suppressing the immune response. It is approved for the treatment of unresectable or metastatic colorectal cancer with dMMR or MSI-H. Pembrolizumab can be given alone or in combination with chemotherapy.
• Nivolumab (Opdivo): Similar to pembrolizumab, nivolumab is a PD-1 inhibitor approved for the treatment of metastatic colorectal cancer with dMMR or MSI-H. It can be used alone or in combination with ipilimumab (Yervoy), a CTLA-4 inhibitor.
• Dostarlimab (Jemperli): Dostarlimab is another PD-1 inhibitor approved for the treatment of recurrent or metastatic colorectal cancer with dMMR.
CTLA-4 Inhibitor
• Ipilimumab (Yervoy): Ipilimumab is a monoclonal antibody that targets the CTLA-4 protein on T cells, enhancing the immune system’s ability to recognize and attack cancer cells. It is often used in combination with nivolumab for the treatment of metastatic colorectal cancer with dMMR or MSI-H.
In addition to these approved immunotherapy drugs, several clinical trials are investigating the use of other immunotherapies, such as cancer vaccines, oncolytic viruses, and adoptive cell therapies, for the treatment of colorectal cancer.
More information about completed and ongoing clinical trials for colorectal cancer can be found here – clinicaltrials.gov
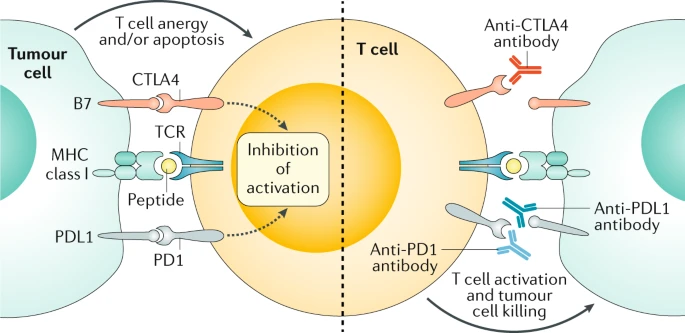
Targets of currently FDA-approved immune checkpoint inhibitors. Image source: Ganesh et al. (2019).
Immunotherapy has emerged as a promising treatment option for colorectal cancer, particularly in cases where standard treatments like chemotherapy and targeted therapy have failed or are no longer effective. However, it is important to note that the efficacy of immunotherapy varies depending on the specific characteristics of the patient’s cancer.
For patients with dMMR or MSI-H colorectal cancer, which accounts for approximately 15% of all colorectal cancer cases, this therapy has shown remarkable results. In a clinical trial (KEYNOTE-177) involving patients with previously untreated metastatic colorectal cancer with dMMR or MSI-H, pembrolizumab demonstrated a significant improvement in survival compared to chemotherapy.
However, for patients with microsatellite stable (MSS) or mismatch repair proficient (pMMR) colorectal cancer, which accounts for the majority of cases, immunotherapy has not been as effective. In these cases, chemotherapy and targeted therapies, such as anti-EGFR (epidermal growth factor receptor) and anti-angiogenic agents, remain the standard of care.
It is important to note that immunotherapy is not a one-size-fits-all solution, and treatment decisions should be made based on the individual patient’s cancer characteristics, overall health, and personal preferences. In some cases, a combination of immunotherapy with other treatment modalities, such as chemotherapy or targeted therapy, may be recommended to achieve the best possible outcomes.
In this video prepared by NEJM, we can see that locally advanced rectal cancer with dMMR showed high sensitivity to single-agent PD-1 blockade.
While immunotherapy has shown promising results in the treatment of colorectal cancer, it can also cause a range of side effects, which can vary in severity and impact. Understanding these side effects is crucial for managing and mitigating them effectively.
Introduction to Immune-Related Adverse Events (irAEs)
IrAEs are side effects that occur when the immune system, activated by immunotherapy, starts attacking normal tissues and organs. These side effects are distinct from those caused by traditional chemotherapy and can affect any part of the body. The most commonly affected organs include the skin, gastrointestinal tract, endocrine organs, liver, joints, and lungs.
• Fatigue: Fatigue is the most common side effect of this treatment. It is usually mild but can be severe in some cases. It is important to rule out underlying causes such as thyroid, pituitary, or adrenal insufficiency.
• Skin Reactions: Skin reactions, including rash, itching, and vitiligo, are common. These reactions are usually mild but can be severe in some cases.
• Gastrointestinal Issues: Diarrhea and colitis (inflammation of the colon) are significant side effects. Symptoms can range from mild diarrhea to severe colitis, which can be life-threatening.
• Endocrine Disorders: Endocrine disorders such as hypothyroidism, hyperthyroidism, adrenal insufficiency, and hypophysitis (inflammation of the pituitary gland) are common.
• Hepatotoxicity: Hepatitis (inflammation of the liver) can occur, leading to elevated liver enzymes.
• Pulmonary Toxicity: Pneumonitis (inflammation of the lungs) is a serious side effect that can cause cough, shortness of breath, and hypoxia.
• Musculoskeletal Issues: These include joint and muscle pain.
• Cardiac Toxicity: Myocarditis (inflammation of the heart muscle) is a rare but potentially fatal side effect. Symptoms include chest pain, arrhythmias, and heart failure.
• Neurological Toxicity: Neurological side effects can include encephalitis (inflammation of the brain), neuropathy (nerve damage), and myasthenia gravis (a neuromuscular disorder).
• Renal Toxicity: Nephritis (inflammation of the kidneys) can lead to renal impairment.
Long-Term Management and Monitoring
Patients who develop endocrinopathies, such as hypothyroidism or adrenal insufficiency, usually require long-term hormone replacement therapy. Regular monitoring of thyroid function, adrenal function, and other relevant parameters is essential. Patients should be educated about the signs and symptoms of irAEs and the importance of reporting them promptly to their healthcare team.
The success of immunotherapy for colorectal cancer largely depends on the specific genetic characteristics of the tumor. For patients with dMMR or MSI-H colorectal cancer, immunotherapy has shown remarkable success, with response rates ranging from 30% to 50%. However, for patients with MSS or pMMR colorectal cancer, which accounts for the majority of cases, immunotherapy has been less effective, with response rates typically below 10%.
Eligibility for immunotherapy in colorectal cancer primarily depends on the genetic characteristics of the tumor. Patients with dMMR or MSI-H colorectal cancer are typically eligible for immunotherapy with immune checkpoint inhibitors like pembrolizumab, nivolumab, and dostarlimab. Additionally, some clinical trials may be exploring immunotherapy options for patients with MSS or pMMR colorectal cancer, or in combination with other treatment modalities.
The percentage of colorectal cancer patients who respond to immunotherapy varies significantly based on the genetic characteristics of the tumor. For patients with dMMR or MSI-H colorectal cancer, which accounts for approximately 15% of all colorectal cancer cases, the response rates to immunotherapy range from 30% to 50%. However, for patients with MSS or pMMR colorectal cancer, which accounts for the majority of cases, the response rates to immunotherapy are typically below 10%.
It is important to note that these response rates are based on clinical trial data and may vary in real-world settings. Additionally, ongoing research and the development of new immunotherapy approaches may improve response rates in the future.
Immunotherapy for Colorectal Cancer – American Cancer Society
Colorectal Cancer Immunotherapy: State of the Art and Future Directions – Gastro Hep Advances
Colorectal Cancer Immunotherapy: Options and Strategies – Frontiers in Immunology
Side Effects of Immunotherapy – The American Society of Clinical Oncology
The article is taken from OncoDaily
About IMMONC
Immune Oncology Research Institute (IMMONC) is dedicated to advancing research aimed at preventing, treating, and ultimately curing cancer while making these innovations accessible to those who need them. If you're interested in joining our team, please feel free to contact us at [email protected] or at +374-41 310-048.