
Kidney cancer, also known as renal cell carcinoma (RCC), is a type of cancer that originates in the kidneys. Immunotherapy is a promising treatment approach that uses the body’s immune system to fight cancer cells. It has emerged as a crucial therapeutic option for renal cancer, particularly in the metastatic setting.
Immunotherapy for kidney cancer aims to stimulate the body’s immune system to recognize and attack cancer cells more effectively. RCC is considered an immunogenic tumor, meaning it can elicit an immune response. However, cancer cells often develop mechanisms to evade immune detection and destruction.
Immunotherapy drugs work by disrupting these evasion mechanisms, allowing the immune system to mount a stronger anti-tumor response. One key mechanism involves blocking immune checkpoint proteins, such as PD-1 (programmed cell death protein 1) and CTLA-4 (cytotoxic T-lymphocyte-associated protein 4), which act as brakes on the immune system. By inhibiting these checkpoints, these drugs release the brakes and enable T cells (a type of immune cell) to recognize and attack cancer cells more effectively. Additionally, some immunotherapy drugs stimulate the immune system directly, enhancing the activity of immune cells against cancer.
Additionally, cytokines like interleukin-2 (IL-2) and interferon-alfa (IFN-α) are used to stimulate and boost the activity of immune cells like T cells and natural killer cells against cancer cells. High-dose IL-2 was one of the earliest immunotherapies approved for kidney cancer, though it has significant toxicity.
Checkpoint inhibitors and cytokines, whether used individually or in combination, are treatment options for advanced or metastatic kidney cancer. Further details about these drugs will be covered in the following section.
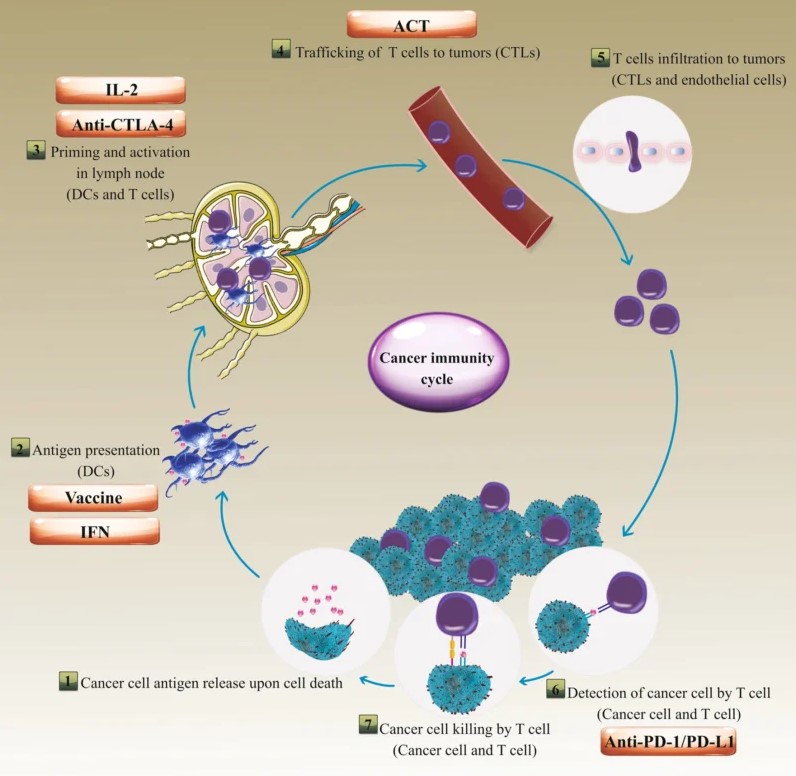
This image is taken from the article Jahangir, M., Yazdani, O., Kahrizi, M.S. et al. Clinical potential of PD-1/PD-L1 blockade therapy for renal cell carcinoma (RCC): a rapidly evolving strategy.
There are several types of immunotherapy drugs approved for the treatment of renal cancer, particularly metastatic renal cell carcinoma. Here’s a look at the different types used in the RCC Clear Cell type:
Immune checkpoint inhibitors are a major class of immunotherapy drugs that work by blocking specific proteins (checkpoints) on immune cells, releasing the brakes on the immune system and allowing it to mount a stronger anti-tumor response.
PD-1 Inhibitors
• Nivolumab (Opdivo): Nivolumab targets the PD-1 protein on T cells, preventing cancer cells from binding to it and inactivating the T cells. It is approved as a single agent and in combination with other drugs for metastatic RCC.
• Pembrolizumab (Keytruda): Like nivolumab, pembrolizumab blocks the PD-1 pathway and is approved for advanced RCC, both as monotherapy and in combination with axitinib (a tyrosine kinase inhibitor).
PD-L1 Inhibitors
• Avelumab (Bavencio): Avelumab targets the PD-L1 protein on cancer cells, preventing it from binding to PD-1 on T cells. It is approved in combination with axitinib for first-line treatment of advanced RCC.
CTLA-4 Inhibitor
• Ipilimumab (Yervoy): Ipilimumab blocks the CTLA-4 protein on T cells, enhancing their activation and proliferation. It is approved in combination with nivolumab for intermediate/poor-risk advanced RCC.
Combining different immunotherapy drugs or immunotherapy with targeted therapies has shown improved efficacy in treating renal cancer.
• Nivolumab + Ipilimumab: This combination of two immune checkpoint inhibitors targeting different pathways is approved for intermediate/poor-risk advanced RCC.
• Pembrolizumab + Axitinib: This combination of a PD-1 inhibitor and a tyrosine kinase inhibitor is approved for first-line treatment of advanced RCC.
• Avelumab + Axitinib: This combination of a PD-L1 inhibitor and a tyrosine kinase inhibitor is also approved for first-line treatment of advanced RCC.
• Nivolumab + Cabozantinib: This combination of a PD-1 inhibitor and a tyrosine kinase inhibitor is approved for first-line treatment of advanced RCC.
• Lenvatinib + Pembrolizumab: This combination of a tyrosine kinase inhibitor and a PD-1 inhibitor is another approved option for first-line treatment of advanced RCC.
Cytokines are proteins that can stimulate the immune system to fight cancer cells. While newer immunotherapies have largely replaced cytokine therapy, some cytokines are still used in certain cases.
• IL-2: High-dose IL-2 can activate T cells and natural killer cells to attack cancer cells. It was one of the earliest immunotherapies approved for metastatic RCC but is now used less frequently due to its toxicity.
• IFN-α: IFN-α can enhance the immune response against cancer cells and was previously used for metastatic RCC, but newer, more effective immunotherapies have largely replaced it.
The choice of immunotherapy drug or combination depends on various factors, including the stage and risk group of the cancer, previous treatments, and the patient’s overall health and preferences. Ongoing research continues to explore novel immunotherapy agents, optimized combinations, and predictive biomarkers to improve outcomes for renal cancer patients further.
This video from the National Kidney Foundation provides a fundamental overview of how immunotherapy treats kidney cancer.
Immunotherapy has revolutionized the treatment landscape for RCC, offering improved outcomes and the potential for durable responses compared to traditional treatment options. However, each modality has its unique strengths and limitations, and the choice depends on various factors, including the stage and risk group of the cancer, patient characteristics, and treatment goals.
Immunotherapy vs. Targeted Therapy
Targeted therapies, such as tyrosine kinase inhibitors (TKIs) and mTOR inhibitors, have been the mainstay of treatment for advanced RCC. However, immunotherapy, particularly immune checkpoint inhibitors, has demonstrated superior efficacy and improved overall survival compared to TKIs in several clinical trials.
Combination regimens involving ICIs and TKIs have become the standard of care for first-line treatment of advanced RCC, offering improved efficacy over TKIs alone.
Immunotherapy vs. Chemotherapy
Chemotherapy has traditionally played a limited role in the treatment of RCC due to the tumor’s inherent chemoresistance. Immunotherapy, particularly ICIs, has demonstrated superior efficacy and a more favorable safety profile compared to chemotherapy in other cancer types, such as non-small cell lung cancer.
While chemotherapy may still be considered in certain situations, such as rapidly progressing or aggressive tumors, it is generally not recommended for the treatment of RCC due to the availability of more effective and better-tolerated options, including immunotherapy and targeted therapies.
Immunotherapy vs. Radiation Therapy
Radiation therapy has been primarily used for palliative purposes in RCC, such as relieving symptoms from bone metastases or brain metastases. However, emerging evidence suggests that radiation therapy can induce immunogenic cell death and release tumor antigens, potentially synergizing with immunotherapy through the abscopal effect (systemic anti-tumor immune response).
Preclinical and early clinical studies have explored the combination of immunotherapy and radiation therapy for RCC, aiming to enhance the anti-tumor immune response and overcome resistance mechanisms.
While immunotherapy is the standard of care for advanced RCC, radiation therapy may play a complementary role, particularly in combination strategies or for local disease control. Ongoing research is investigating the optimal integration of these modalities to further improve outcomes.
It’s important to note that treatment decisions are highly individualized and often involve a multimodal approach, combining different treatment modalities based on the patient’s specific circumstances, cancer type, and overall health status.
Information about finished and ongoing clinical trials for Renal Cell Carcinoma can be found here clinicaltrials.gov
While immunotherapy has revolutionized the treatment of renal cancer, it can also cause side effects due to the activation of the immune system. Common side effects include:
• Fatigue
• Skin rash
• Diarrhea
• Nausea and vomiting
• Decreased appetite
• Cough
• Hypothyroidism (underactive thyroid)
• Pneumonitis (inflammation of the lungs)
In some cases, it can lead to more severe immune-related adverse events (irAEs), such as:
• Colitis (inflammation of the colon)
• Hepatitis (liver inflammation)
• Nephritis (kidney inflammation)
• Endocrinopathies (hormone imbalances)
• Neurological complications
These side effects can range from mild to severe and may require treatment interruption, dose modification, or the use of immunosuppressive medications like corticosteroids.
Immunotherapy has significantly improved outcomes for patients with metastatic RCC. Clinical trials have shown that immunotherapy, particularly in combination with targeted therapies, can prolong overall survival and progression-free survival compared to traditional treatments. However, individual responses may vary, and ongoing research aims to identify predictive biomarkers to better select patients who are most likely to benefit from this approach.
As of 2024, the latest approved treatment options for renal cancer include:
• Pembrolizumab as adjuvant (post-surgery) therapy for high-risk RCC patients, has shown improved overall survival compared to placebo.
• Combination therapies such as nivolumab + cabozantinib, lenvatinib + pembrolizumab, and avelumab + axitinib as first-line treatments for metastatic RCC, regardless of risk group or PD-L1 expression.
Additionally, ongoing clinical trials are evaluating novel immunotherapy agents and combinations, aiming to further improve outcomes for renal cancer patients.
Eligibility for immunotherapy in renal cancer depends on various factors, including the stage and subtype of the cancer, the patient’s overall health, and treatment goals. Generally, immunotherapy is recommended for patients with metastatic or advanced RCC, either as a first-line treatment or after progression on other therapies. Patients with certain autoimmune disorders or organ transplant recipients may not be eligible due to the risk of exacerbating autoimmune reactions or graft rejection. Close monitoring and careful patient selection are crucial for optimal outcomes.
Immunotherapy for kidney cancer – Canadian Cancer Society
Renal cell Carcinoma Clinical Trials – clinicaltrials.gov
Targeted and immunotherapy drugs for advanced kidney cancer – Cancer Research UK
The article is taken from OncoDaily
About IMMONC
Immune Oncology Research Institute (IMMONC) is dedicated to advancing research aimed at preventing, treating, and ultimately curing cancer while making these innovations accessible to those who need them. If you're interested in joining our team, please feel free to contact us at [email protected] or at +374-41 310-048.